EP-307 Introduction to Quantum Mechanics
... if you do an experiment it should tell you whether it will behave as a wave or particle. Second Question brings us to the domain of the theory ...
... if you do an experiment it should tell you whether it will behave as a wave or particle. Second Question brings us to the domain of the theory ...
chapter 7 part 2
... some arrangements, introducing constants ml and l(l+1) – which will later on turn out to be significant - we finally end up with ...
... some arrangements, introducing constants ml and l(l+1) – which will later on turn out to be significant - we finally end up with ...
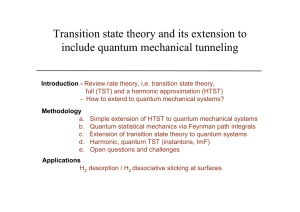
Transition state theory and its extension to include quantum
... How can dynamical corrections be evaluated given a RAW quantum mechanical ...
... How can dynamical corrections be evaluated given a RAW quantum mechanical ...
III. Quantum Model of the Atom
... Applied wave-particle theory to ee- exhibit wave properties QUANTIZED WAVELENGTHS ...
... Applied wave-particle theory to ee- exhibit wave properties QUANTIZED WAVELENGTHS ...
A1980KM40500001
... transformed so that, for a completely unsymmetrical molecule, there are only (n+ 1) terms for each even degree n. [The SCI ® indicates that this paper has been cited over 305 times since 1967.] James K. G. Watson Department of Chemistry University of Southampton Southampton SO9 5NH England September ...
... transformed so that, for a completely unsymmetrical molecule, there are only (n+ 1) terms for each even degree n. [The SCI ® indicates that this paper has been cited over 305 times since 1967.] James K. G. Watson Department of Chemistry University of Southampton Southampton SO9 5NH England September ...
Chapter 5
... Atomic Emission Spectrum Spectrometer: Breaks up what we see as continuous light into individual bands of light. The individual bands of light represent the exact frequency of light be given off. This corresponds to the quantum of energy that is released when an electron goes from an excited state ...
... Atomic Emission Spectrum Spectrometer: Breaks up what we see as continuous light into individual bands of light. The individual bands of light represent the exact frequency of light be given off. This corresponds to the quantum of energy that is released when an electron goes from an excited state ...
The Boltzmann equation
... This approach permits to find all the known results in the collisionless or hydrodynamic regime, it gives an interpolation from the collisionless regime to the hydrodynamic regime. Consistent with numerical simulations. Recently generalized to include Fermi statistics EuroPhys. Lett. 67, 534 (2004) ...
... This approach permits to find all the known results in the collisionless or hydrodynamic regime, it gives an interpolation from the collisionless regime to the hydrodynamic regime. Consistent with numerical simulations. Recently generalized to include Fermi statistics EuroPhys. Lett. 67, 534 (2004) ...
Lecture 33
... This was true in classical physics. Now we want to extend the analogy to quantum physics. A quantum field should be like an infinite number of quantum particles. Each of those is a wave packet (Lect. 23), which again contains an infinite number of points. ...
... This was true in classical physics. Now we want to extend the analogy to quantum physics. A quantum field should be like an infinite number of quantum particles. Each of those is a wave packet (Lect. 23), which again contains an infinite number of points. ...
10mod_phys
... – Used quantum theory, and atomic spectra to fix problems with the Rutherford model. Proposed: – An electron can only occupy certain allowed orbits without radiating – Each nth orbit has a radius (rn) and an energy (En). – An electron can make a transition between two orbits through • Absorbing a Ph ...
... – Used quantum theory, and atomic spectra to fix problems with the Rutherford model. Proposed: – An electron can only occupy certain allowed orbits without radiating – Each nth orbit has a radius (rn) and an energy (En). – An electron can make a transition between two orbits through • Absorbing a Ph ...
God Plays Dice
... • I think it is safe to say that no one understands quantum mechanics. Do not keep saying to yourself, if you can possibly avoid it, 'But how can it possibly be like that?' because you will go down the drain into a blind alley from which nobody has yet escaped. Nobody knows how it can be like that. ...
... • I think it is safe to say that no one understands quantum mechanics. Do not keep saying to yourself, if you can possibly avoid it, 'But how can it possibly be like that?' because you will go down the drain into a blind alley from which nobody has yet escaped. Nobody knows how it can be like that. ...
Particle in a box

In quantum mechanics, the particle in a box model (also known as the infinite potential well or the infinite square well) describes a particle free to move in a small space surrounded by impenetrable barriers. The model is mainly used as a hypothetical example to illustrate the differences between classical and quantum systems. In classical systems, for example a ball trapped inside a large box, the particle can move at any speed within the box and it is no more likely to be found at one position than another. However, when the well becomes very narrow (on the scale of a few nanometers), quantum effects become important. The particle may only occupy certain positive energy levels. Likewise, it can never have zero energy, meaning that the particle can never ""sit still"". Additionally, it is more likely to be found at certain positions than at others, depending on its energy level. The particle may never be detected at certain positions, known as spatial nodes.The particle in a box model provides one of the very few problems in quantum mechanics which can be solved analytically, without approximations. This means that the observable properties of the particle (such as its energy and position) are related to the mass of the particle and the width of the well by simple mathematical expressions. Due to its simplicity, the model allows insight into quantum effects without the need for complicated mathematics. It is one of the first quantum mechanics problems taught in undergraduate physics courses, and it is commonly used as an approximation for more complicated quantum systems.