MODULE DESCRIPTOR Code: Alt Codes: Title:
... Lectures and tutorials. Tutorial sheets and other exercises will be used to provide formative assessment. Practical classes related to the reinforcement of conceptual ideas taught in this subject will be conducted and assessed in MECH102P Mechanical Engineering Practical Skills I. Assessment: Writte ...
... Lectures and tutorials. Tutorial sheets and other exercises will be used to provide formative assessment. Practical classes related to the reinforcement of conceptual ideas taught in this subject will be conducted and assessed in MECH102P Mechanical Engineering Practical Skills I. Assessment: Writte ...
8.8.b Conservation of Energy 2
... of Energy to different situations in an effort to calculate unknown variables. ...
... of Energy to different situations in an effort to calculate unknown variables. ...
W - crashwhite.com
... The Law of Conservation of Energy states that energy cannot be created or destroyed. This means that the total energy in an isolated system doesn’t change over time. We can use this principle to determine how energy transforms from form to form throughout the various systems we observe. It’s espec ...
... The Law of Conservation of Energy states that energy cannot be created or destroyed. This means that the total energy in an isolated system doesn’t change over time. We can use this principle to determine how energy transforms from form to form throughout the various systems we observe. It’s espec ...
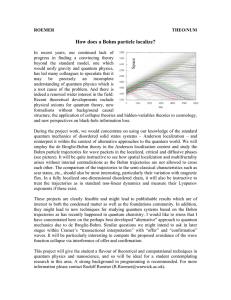
How does a Bohm particle localize?
... arises without internal contradictions as the Bohm trajectories are not allowed to cross each other. The comparison of the trajectories to the semi-classical characteristics such as scar states, etc., should also be most interesting, particularly their variation with magnetic flux. In a fully locali ...
... arises without internal contradictions as the Bohm trajectories are not allowed to cross each other. The comparison of the trajectories to the semi-classical characteristics such as scar states, etc., should also be most interesting, particularly their variation with magnetic flux. In a fully locali ...
F - Aurora City Schools
... with its position in a gravitational field (work done against the force of gravity to put it there) ...
... with its position in a gravitational field (work done against the force of gravity to put it there) ...
Text sections 27.4, 27.6, 28.1 • Drude Model • Electrical work and
... The average kinetic energy of the electrons doesn’t increase once the current reaches a steady state; the electrons lose energy in collisions as fast as it is supplied by the field. Negative charge carriers would move in the opposite direction (from low V to higher V); but this still means they lose ...
... The average kinetic energy of the electrons doesn’t increase once the current reaches a steady state; the electrons lose energy in collisions as fast as it is supplied by the field. Negative charge carriers would move in the opposite direction (from low V to higher V); but this still means they lose ...
EEP_Syllabus - Classical Physics / Santurri : Students
... At appropriate points in the course, the investigations listed below will be presented to the students to test and verify the physical principles being studies. Often a demonstration of a physical phenomenon will be presented to the class and an explanation of the event will be requested. Students w ...
... At appropriate points in the course, the investigations listed below will be presented to the students to test and verify the physical principles being studies. Often a demonstration of a physical phenomenon will be presented to the class and an explanation of the event will be requested. Students w ...
Document
... The Bohr Model was an early attempt to formulate a quantum theory of matter Niels Bohr made the following postulates for a single e − orbiting a nucleus of Z protons: 1. The e − moves in a circular orbit around the nucleus. 2. The energy of the e − can take on only certain well-defined values, that ...
... The Bohr Model was an early attempt to formulate a quantum theory of matter Niels Bohr made the following postulates for a single e − orbiting a nucleus of Z protons: 1. The e − moves in a circular orbit around the nucleus. 2. The energy of the e − can take on only certain well-defined values, that ...
Chapter 5.1 Models of the Atom
... Atomic orbital: region of space where there is a high probability of finding an electron ...
... Atomic orbital: region of space where there is a high probability of finding an electron ...
Momentum
... Example: A 1.0 kg mass is held 10 m above the ground. Find its G.P.E. relative to the ground. [U = 1.0 kg ( 9.8 ms–2 ( 10 m = 98 J] ...
... Example: A 1.0 kg mass is held 10 m above the ground. Find its G.P.E. relative to the ground. [U = 1.0 kg ( 9.8 ms–2 ( 10 m = 98 J] ...
Chapter 5 Notes
... Bohr’s model was the foundation for atomic models that came later, but his model was actually wrong because electrons do not move around the nucleus in orbits. ...
... Bohr’s model was the foundation for atomic models that came later, but his model was actually wrong because electrons do not move around the nucleus in orbits. ...
introduction to energy* worksheet
... Part 3. Forms of Energy. Directions: Determine the type of energy for each form (Kinetic, Potential, or Both) and give an example. Form Definition Type (KE, PE, Example (for each or Both) type if both) Mechanical (motion) energy Thermal (heat) energy Radiant energy Electrical energy Chemical energy ...
... Part 3. Forms of Energy. Directions: Determine the type of energy for each form (Kinetic, Potential, or Both) and give an example. Form Definition Type (KE, PE, Example (for each or Both) type if both) Mechanical (motion) energy Thermal (heat) energy Radiant energy Electrical energy Chemical energy ...
Ch 14, 15, 16
... needed to raise the temp of 1g of a material by 1oC • Think of it as “resistance to temperature change” • The higher the c, the harder it is the change the temp. ...
... needed to raise the temp of 1g of a material by 1oC • Think of it as “resistance to temperature change” • The higher the c, the harder it is the change the temp. ...