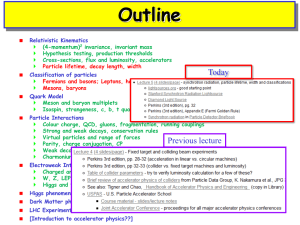
Lecture 4/5 - TCD Chemistry
... First Law of Thermodynamics: Internal energy U or E. Internal energy (U or E) : is the total energy of the system at any given time. Comes from the total kinetic and potential energy of molecules which compose the system. Change in internal energy (U or E) : energy change as system goes from an i ...
... First Law of Thermodynamics: Internal energy U or E. Internal energy (U or E) : is the total energy of the system at any given time. Comes from the total kinetic and potential energy of molecules which compose the system. Change in internal energy (U or E) : energy change as system goes from an i ...
The elastic potential energy
... A bullet of mass m travelling horizontally with speed u strikes perpendicularly a wooden block of mass M which is free to move. The penetration is assumed uniform and the bullet comes to rest after penetrating a distance a into the block. Show that the ultimate common velocity of the system is penet ...
... A bullet of mass m travelling horizontally with speed u strikes perpendicularly a wooden block of mass M which is free to move. The penetration is assumed uniform and the bullet comes to rest after penetrating a distance a into the block. Show that the ultimate common velocity of the system is penet ...
LAB 5
... Using your energy calibration curve from Lab 4 roughly how many voltage bins does this energy spread (E) cover? Could you possibly ever see this energy spread using the equipment that we have in this lab? (II) Measure the energy resolution of your NaI detector using Co60, Na22, and Cs137. Take a sp ...
... Using your energy calibration curve from Lab 4 roughly how many voltage bins does this energy spread (E) cover? Could you possibly ever see this energy spread using the equipment that we have in this lab? (II) Measure the energy resolution of your NaI detector using Co60, Na22, and Cs137. Take a sp ...
Conservation of Energy
... In this exercise the variation of the final velocity of the cart and hanging mass as a function of the initial height will be measured experimentally. 1. Untie the mass hanger from the glider. Measure and record the mass of the glider as Mc . 2. Place the glider on the air track and turn the air sup ...
... In this exercise the variation of the final velocity of the cart and hanging mass as a function of the initial height will be measured experimentally. 1. Untie the mass hanger from the glider. Measure and record the mass of the glider as Mc . 2. Place the glider on the air track and turn the air sup ...
Kinetic and Potential Energy Answers
... Have you ever thought about.....why you are able to get out of bed in the morning and come to school? Why the alarm clock sounds in the morning? How the toaster toasts bread? How your car runs? How you run? Why your nose runs? How your computer runs? How everything runs? NO, you say? WHY NOT?????? D ...
... Have you ever thought about.....why you are able to get out of bed in the morning and come to school? Why the alarm clock sounds in the morning? How the toaster toasts bread? How your car runs? How you run? Why your nose runs? How your computer runs? How everything runs? NO, you say? WHY NOT?????? D ...
HW7 solutions - Itai Cohen
... about its equilibrium position. Calculate the heat capacity of this system of particles at this temperature in each of the following cases: (a) The force effective in resotring each particle to its equilibrium position is proportional to its displacement x from this position. (b) The restoring force ...
... about its equilibrium position. Calculate the heat capacity of this system of particles at this temperature in each of the following cases: (a) The force effective in resotring each particle to its equilibrium position is proportional to its displacement x from this position. (b) The restoring force ...
kinetic energy
... According to this Law, energy is never created or destroyed, it can only _______________. What is the name of the law from #3 Energy of movement is called __________ energy. Energy that comes from food is measured in ________. Potential Energy and Kinetic energy is measured in ___. At the top of the ...
... According to this Law, energy is never created or destroyed, it can only _______________. What is the name of the law from #3 Energy of movement is called __________ energy. Energy that comes from food is measured in ________. Potential Energy and Kinetic energy is measured in ___. At the top of the ...
Summary
... Sunlight is directly transformed into electricity by photovoltaic cells or in the flexible solar shingles on the roofs of buildings. We use the energy in sunlight to generate electricity indirectly as well. Wind, caused by unequal warming of Earth’s surface, is another form of solar power. Wind can ...
... Sunlight is directly transformed into electricity by photovoltaic cells or in the flexible solar shingles on the roofs of buildings. We use the energy in sunlight to generate electricity indirectly as well. Wind, caused by unequal warming of Earth’s surface, is another form of solar power. Wind can ...
Properties of Matter - Broadneck High School
... Temperature Scales • Temperature can be subjective and so fixed scales had to be introduced. • The boiling point and freezing point of water are two such points. • Celsius scale (oC) – The Celsius scale divides the range from freezing to boiling into 100 divisions. – Original scale had freezing as ...
... Temperature Scales • Temperature can be subjective and so fixed scales had to be introduced. • The boiling point and freezing point of water are two such points. • Celsius scale (oC) – The Celsius scale divides the range from freezing to boiling into 100 divisions. – Original scale had freezing as ...
1991B5 A polonium nucleus of atomic number 84
... What is the velocity of the alpha particle? (Neglect relativistic effects for this calculation.) c. Where does the kinetic energy of the alpha particle come from? Explain briefly. d. Suppose that the fermium-252 nucleus could undergo a decay in which a β- particle was produced. How would this affect ...
... What is the velocity of the alpha particle? (Neglect relativistic effects for this calculation.) c. Where does the kinetic energy of the alpha particle come from? Explain briefly. d. Suppose that the fermium-252 nucleus could undergo a decay in which a β- particle was produced. How would this affect ...
Energy Types and Forms
... • A buildup of electrons in one place will create a force that pushes the electrons away • This force causes a voltage or potential energy • If the electrons are able to move, the potential energy will cause a current or kinetic energy in the electrons ...
... • A buildup of electrons in one place will create a force that pushes the electrons away • This force causes a voltage or potential energy • If the electrons are able to move, the potential energy will cause a current or kinetic energy in the electrons ...
Physics 101
... Two masses 3 meters from each other exert a gravitational force of 300 Newtons on each other. If those same two masses were brought closer to a distance of 1 m from each other, what would be the force of attraction between them? a) 100 N b) 300 N c) 2700 N d) 8100 N ...
... Two masses 3 meters from each other exert a gravitational force of 300 Newtons on each other. If those same two masses were brought closer to a distance of 1 m from each other, what would be the force of attraction between them? a) 100 N b) 300 N c) 2700 N d) 8100 N ...