C10J ATOMIC STRUCTURE (6 lectures)
... The Atomic Structure course is considered as an important part of the core course for Introductory Chemistry as concepts which are learnt here will be employed in the subsequent sections on Physical, Inorganic (e.g. bonding and antibonding orbitals) and Organic Chemistry (e.g. hybridization). This c ...
... The Atomic Structure course is considered as an important part of the core course for Introductory Chemistry as concepts which are learnt here will be employed in the subsequent sections on Physical, Inorganic (e.g. bonding and antibonding orbitals) and Organic Chemistry (e.g. hybridization). This c ...
NTD_Final_Ch3-1_3-2 DOWNLOAD
... electrons can be lost by thermal excitation leaving behind an unsaturated bond or hole. In the silicon doped by an element in group 15 of the periodic table (e.g. phosphorus, arsenic, or antimony), some silicon atoms in the lattice are substituted by the dopant atoms as shown in Fig. 2(b). Because t ...
... electrons can be lost by thermal excitation leaving behind an unsaturated bond or hole. In the silicon doped by an element in group 15 of the periodic table (e.g. phosphorus, arsenic, or antimony), some silicon atoms in the lattice are substituted by the dopant atoms as shown in Fig. 2(b). Because t ...
Investigation of excitation energies and Hund`s rule in open shell
... 3.1 Ground state results and Hund’s first rules In Table 1 we show ground state DMC and VMC energies. The DMC and VMC ground states of both dots have L = 0, S = 2 symmetry. The total spin S is the maximum allowed for four open-shell electrons, complying with Hund’s first rule. For the N = 24 case, i ...
... 3.1 Ground state results and Hund’s first rules In Table 1 we show ground state DMC and VMC energies. The DMC and VMC ground states of both dots have L = 0, S = 2 symmetry. The total spin S is the maximum allowed for four open-shell electrons, complying with Hund’s first rule. For the N = 24 case, i ...
The fractional quantum Hall effect: Laughlin wave function, fractional
... PHYS598PTD A.J.Leggett ...
... PHYS598PTD A.J.Leggett ...
2.8 M - Thierry Karsenti
... Atomic physics may loosely be defined as the scientific study of the structure of the atom, its energy states, and its interactions with other particles and fields. Learning Atomic Physics is important not only for understanding the physics of the atom but also the technological applications thereof ...
... Atomic physics may loosely be defined as the scientific study of the structure of the atom, its energy states, and its interactions with other particles and fields. Learning Atomic Physics is important not only for understanding the physics of the atom but also the technological applications thereof ...
SPIN-LIQUIDS ON THE KAGOME LATTICE: CHIRAL
... (Non-interacting Majorana Fermion Toy model) Think in terms of a “network model” to try to gain intuition about the behavior of the system: ...
... (Non-interacting Majorana Fermion Toy model) Think in terms of a “network model” to try to gain intuition about the behavior of the system: ...
Chapter 39 - KFUPM Faculty List
... 39.4.4. What is the zero-point energy? a) The smallest amount of energy that any particle can have in the Universe is called the zero-point energy. b) The smallest energy that an electron confined within an atom is zero joules, which is the zero point energy. c) The energy that an electron has at t ...
... 39.4.4. What is the zero-point energy? a) The smallest amount of energy that any particle can have in the Universe is called the zero-point energy. b) The smallest energy that an electron confined within an atom is zero joules, which is the zero point energy. c) The energy that an electron has at t ...
1. Structure of Matter
... quantized. Bohr noted that this quantization nicely explained the observed emission spectrum of the hydrogen atom. The electron is normally in its smallest allowed orbit, corresponding to n = 1; upon excitation in an electrical discharge or by ultraviolet light, the atom absorbs energy and the elect ...
... quantized. Bohr noted that this quantization nicely explained the observed emission spectrum of the hydrogen atom. The electron is normally in its smallest allowed orbit, corresponding to n = 1; upon excitation in an electrical discharge or by ultraviolet light, the atom absorbs energy and the elect ...
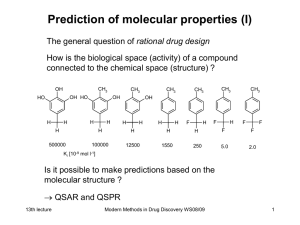
Modern Methods in Drug Discovery
... As a simplification the wave function of all electrons in a molecule is assumed to be the product of one-electron functions which themselves describe a single electron. ...
... As a simplification the wave function of all electrons in a molecule is assumed to be the product of one-electron functions which themselves describe a single electron. ...
X-ray Diffraction
... X-rays are short wavelength photons (0.1Å to 10Å), usually produced when high-energy electrons interact with solid matter. The electrons lose energy by collisions with the atoms and much of the lost energy is radiated as photons. (The rest of the energy is dissipated as heat in the solid material, s ...
... X-rays are short wavelength photons (0.1Å to 10Å), usually produced when high-energy electrons interact with solid matter. The electrons lose energy by collisions with the atoms and much of the lost energy is radiated as photons. (The rest of the energy is dissipated as heat in the solid material, s ...
Electronic Structure of Multi-Electron Quantum Dots
... using Slater determinants composed of single-electron eigenstates as the basis functions. This approach, namely the configuration interaction (CI) method, takes into account the full interaction and correlation of the electrons in the system as long as the numerical results converge with an increasi ...
... using Slater determinants composed of single-electron eigenstates as the basis functions. This approach, namely the configuration interaction (CI) method, takes into account the full interaction and correlation of the electrons in the system as long as the numerical results converge with an increasi ...
Chapter 3 Statistical thermodynamics
... It can be seen from the formulas above U, H and the expression of Cv are the same in the localized and non-localized system; However, in the expressions of F, S and G, compared with the localized system, it lacks the relational 1/N! constant, but it can be expurgated each other when we calculate the ...
... It can be seen from the formulas above U, H and the expression of Cv are the same in the localized and non-localized system; However, in the expressions of F, S and G, compared with the localized system, it lacks the relational 1/N! constant, but it can be expurgated each other when we calculate the ...
Simple Theory of the Magnetic Properties of Rare
... The same zeroth-order Hamiltonian has been used successfully' to explain the n-y phase transition in cerium metal. It assumes that the levels and the conduction band are not hybridized. ...
... The same zeroth-order Hamiltonian has been used successfully' to explain the n-y phase transition in cerium metal. It assumes that the levels and the conduction band are not hybridized. ...
Bohr Theory in the Atomic Physics
... Bohr Theory is one important stage in the development of the theory of atomic physics, and it has achieved great achievements when dealing with the problem of hydrogen atom and H-like ion, and it is on the important status in the teaching of atomic physics. Combining with teaching experiences, the h ...
... Bohr Theory is one important stage in the development of the theory of atomic physics, and it has achieved great achievements when dealing with the problem of hydrogen atom and H-like ion, and it is on the important status in the teaching of atomic physics. Combining with teaching experiences, the h ...
Interactions and Interference in Quantum Dots: Kinks in Coulomb
... positions: Gaussian process simulation results as a function of scaled parameter X (β = 2). (a) 3 typical orbital energy levels; note the infrequent avoided crossings. (b) Two sets of energy levels, one for singly occupied orbitals (solid) and the second for double occupancy (dashed). For a large do ...
... positions: Gaussian process simulation results as a function of scaled parameter X (β = 2). (a) 3 typical orbital energy levels; note the infrequent avoided crossings. (b) Two sets of energy levels, one for singly occupied orbitals (solid) and the second for double occupancy (dashed). For a large do ...