N/Z = 2, 8, 20, 28, 50, 82, 126
... Systematics are reminiscent of the periodic structure of atoms, which results from filling independent single-particle electron states with electrons in the most efficient way consistent with the Pauli principle, but the magic numbers are different: “Magic numbers” for atoms: Z = 2, 10, 18, 30, 36, ...
... Systematics are reminiscent of the periodic structure of atoms, which results from filling independent single-particle electron states with electrons in the most efficient way consistent with the Pauli principle, but the magic numbers are different: “Magic numbers” for atoms: Z = 2, 10, 18, 30, 36, ...
Atomic Emission Spectra – Copy
... impossible to know precisely both the velocity and position of a particle at the same time. • In other words, the light used to measure the particle changes it. • Now watch the Captain Quantum video. You can find it on my website on the assignments page. ...
... impossible to know precisely both the velocity and position of a particle at the same time. • In other words, the light used to measure the particle changes it. • Now watch the Captain Quantum video. You can find it on my website on the assignments page. ...
qp2
... they spin that they spin in opposite directions. Hence electrons keep their distance and lead to atomic sizes as we see. This amazing principle explains why matter doesn't bunch up into a small space and therefore why we (and the Universe) exist without imploding on ourselves. Riddle of disappearanc ...
... they spin that they spin in opposite directions. Hence electrons keep their distance and lead to atomic sizes as we see. This amazing principle explains why matter doesn't bunch up into a small space and therefore why we (and the Universe) exist without imploding on ourselves. Riddle of disappearanc ...
An Introduction to the Concept of Band Structure
... are degenerate in k (i.e. flat) at the energies of the quantum well levels, while the width is continuously increasing with the overlap of the wave functions (or decreasing barrier width between the wells). This is illustrated in Fig. 5(c). These features hold also in higher dimensions, and are very ...
... are degenerate in k (i.e. flat) at the energies of the quantum well levels, while the width is continuously increasing with the overlap of the wave functions (or decreasing barrier width between the wells). This is illustrated in Fig. 5(c). These features hold also in higher dimensions, and are very ...
Chapter 5
... groundwork for atomic models to come, but was later disproved because it did not fully account for the chemical behavior of atoms. ...
... groundwork for atomic models to come, but was later disproved because it did not fully account for the chemical behavior of atoms. ...
Lesson7
... away by the fragments as kinetic energy, which is not quantized. • The atomic products can be in an excited state. ...
... away by the fragments as kinetic energy, which is not quantized. • The atomic products can be in an excited state. ...
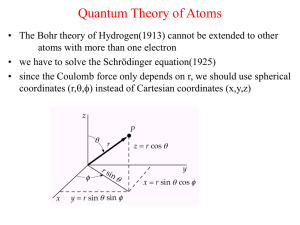
Quantum Theory of Atoms
... Quantum Theory of Atoms • The Bohr theory of Hydrogen(1913) cannot be extended to other atoms with more than one electron • we have to solve the Schrödinger equation(1925) • since the Coulomb force only depends on r, we should use spherical coordinates (r,,) instead of Cartesian coordinates (x,y,z ...
... Quantum Theory of Atoms • The Bohr theory of Hydrogen(1913) cannot be extended to other atoms with more than one electron • we have to solve the Schrödinger equation(1925) • since the Coulomb force only depends on r, we should use spherical coordinates (r,,) instead of Cartesian coordinates (x,y,z ...
Laboratory 1
... 1. Plot the window function for the phonon transport 2 at different temperatures. k T ...
... 1. Plot the window function for the phonon transport 2 at different temperatures. k T ...
Density Matrix
... state variables are microscopic, and a state is specified by giving the positions and velocities of all particles as a function of time. Obviously, this does not generalize to the quantum theory very easily, thus in our work we will try to adhere as closely as possible to the first meaning, but it n ...
... state variables are microscopic, and a state is specified by giving the positions and velocities of all particles as a function of time. Obviously, this does not generalize to the quantum theory very easily, thus in our work we will try to adhere as closely as possible to the first meaning, but it n ...
Solving the Helium Atom
... resources, the calculated energies disagreed with reality by a reasonably large amount. In virtually all reasonable (e.g. L greater than 5) cases, the value for the first ionized state was calculated to be the same value independent of parameters: –54.46eV. This compares to a true value of –54.42eV, ...
... resources, the calculated energies disagreed with reality by a reasonably large amount. In virtually all reasonable (e.g. L greater than 5) cases, the value for the first ionized state was calculated to be the same value independent of parameters: –54.46eV. This compares to a true value of –54.42eV, ...
Department of Physical Sciences (Physics)
... from the sun ejects electrons from their outer surface and they must be designed to minimise this effect. If the skin is coated with Ni which has a work function of 4.87eV, calculate the longest wavelength of the incident sunlight which can eject a photoelectron. With this in mind comment on whether ...
... from the sun ejects electrons from their outer surface and they must be designed to minimise this effect. If the skin is coated with Ni which has a work function of 4.87eV, calculate the longest wavelength of the incident sunlight which can eject a photoelectron. With this in mind comment on whether ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 16. (a) Explain the structure of space-time (Minkowski) diagram and bring out its salient features. (b) The coordinates of event A are ( x A, 0, 0, t A) and the coordinates of event B are ( x B, 0, 0, t B). Assuming the interval between them is space- like, find the velocity of the system in which t ...
... 16. (a) Explain the structure of space-time (Minkowski) diagram and bring out its salient features. (b) The coordinates of event A are ( x A, 0, 0, t A) and the coordinates of event B are ( x B, 0, 0, t B). Assuming the interval between them is space- like, find the velocity of the system in which t ...
Atomic Orbitals Lab - North Carolina High School Computational
... for predicting the behaviors of a number of atomic properties. Unfortunately, it also has some significant problems as a model for atomic structure. ...
... for predicting the behaviors of a number of atomic properties. Unfortunately, it also has some significant problems as a model for atomic structure. ...
Chemistry 330 Chapter 11
... Energy of the H atom is quantized Electron is promoted from a low to high energy level by the absorption of a photon The amount of energy absorbed and emitted by the atom is quantized Only orbits of certain angular momenta are allowed ...
... Energy of the H atom is quantized Electron is promoted from a low to high energy level by the absorption of a photon The amount of energy absorbed and emitted by the atom is quantized Only orbits of certain angular momenta are allowed ...