Chapter 7 Quantum Theory of the Atom
... Magnetic Quantum Number, ml This quantum number distinguishes orbitals of a given n and l—that is, of a given energy and shape but having different orientations. The magnetic quantum number depends on the value of l and can have any integer value from –l to 0 to +l. Each different value represents ...
... Magnetic Quantum Number, ml This quantum number distinguishes orbitals of a given n and l—that is, of a given energy and shape but having different orientations. The magnetic quantum number depends on the value of l and can have any integer value from –l to 0 to +l. Each different value represents ...
Lectuer 15
... on both n and Ɩ. - If Ɩ = 0 being denoted by s, Ɩ = 1 by p, Ɩ = 2 by d, Ɩ = 3 by f - The letter s, p, d, and f stand for sharp, principal, diffuse, and fundamental. - A wave function with n = 1 and Ɩ = 0 is called 1s wave function; one with n =2 and Ɩ = 0 a 2s wave function, and so on. ...
... on both n and Ɩ. - If Ɩ = 0 being denoted by s, Ɩ = 1 by p, Ɩ = 2 by d, Ɩ = 3 by f - The letter s, p, d, and f stand for sharp, principal, diffuse, and fundamental. - A wave function with n = 1 and Ɩ = 0 is called 1s wave function; one with n =2 and Ɩ = 0 a 2s wave function, and so on. ...
1 The Photoelectric Effect 2 Line Spectra and Energy Levels
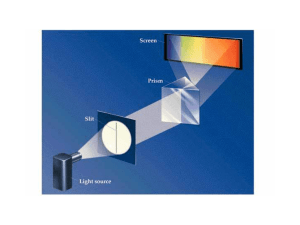
... Bohr model : a mechanical model of the hydrogen atom proposed by Bohr using the relationship between spectral wavelengths and energy levels. stable orbits: path of the electron revolving around the nucleus that do not decay and do not emit radiation (contrary to classical electromagnetic theory). qu ...
... Bohr model : a mechanical model of the hydrogen atom proposed by Bohr using the relationship between spectral wavelengths and energy levels. stable orbits: path of the electron revolving around the nucleus that do not decay and do not emit radiation (contrary to classical electromagnetic theory). qu ...
MSWORD document
... structure sequences are generated during the optimization procedures at various steps till the end. And enquiring into the structural details and the sequences of structures obtained during the Geometry Optimization can be providing indications to the various pathways of interactions of cluster comp ...
... structure sequences are generated during the optimization procedures at various steps till the end. And enquiring into the structural details and the sequences of structures obtained during the Geometry Optimization can be providing indications to the various pathways of interactions of cluster comp ...
Chapter 2 - UCF Chemistry
... • Bohr’s theory correctly explains the H emission spectrum and those of hydrogenlike ions (He+, Li2+ … 1e− species) • The theory fails for atoms of all other elements because it is not an adequate theory: it doesn’t take into account the fact that the (very small) electron can be thought as having w ...
... • Bohr’s theory correctly explains the H emission spectrum and those of hydrogenlike ions (He+, Li2+ … 1e− species) • The theory fails for atoms of all other elements because it is not an adequate theory: it doesn’t take into account the fact that the (very small) electron can be thought as having w ...
Lecture 9-21-11a
... distance of the electron from the nucleus same as n for the Bohr atom ℓangular momentum q. n. - shape of the orbital mℓmagnetic q. n. - orientation in space CHEM131 - Fall 11 - September 21 ...
... distance of the electron from the nucleus same as n for the Bohr atom ℓangular momentum q. n. - shape of the orbital mℓmagnetic q. n. - orientation in space CHEM131 - Fall 11 - September 21 ...
Slide 1
... Photoelectric Effect: electrons are ejected from a metal's surface if it is exposed to uv radiation Each metal required a characteristic minimum uv frequency to start ejecting e-s Called Threshold freq, no - As n increases more e-s ejected with higher vel (KE) These data also defied classical physic ...
... Photoelectric Effect: electrons are ejected from a metal's surface if it is exposed to uv radiation Each metal required a characteristic minimum uv frequency to start ejecting e-s Called Threshold freq, no - As n increases more e-s ejected with higher vel (KE) These data also defied classical physic ...
Notations for today’s lecture (1 ) A complete set of ;
... Φα({x} ; t ) = < 0 | eitH/ħ Ψ(x1)Ψ(x2)...Ψ(xN)e−itH/ħ | α > (Trick question: Is this the Schroedinger picture or the Heisenberg picture?) Note that Φα is not an expectation value. The N factors of Ψ annihilate the particles, ...
... Φα({x} ; t ) = < 0 | eitH/ħ Ψ(x1)Ψ(x2)...Ψ(xN)e−itH/ħ | α > (Trick question: Is this the Schroedinger picture or the Heisenberg picture?) Note that Φα is not an expectation value. The N factors of Ψ annihilate the particles, ...
lecture 21 - UMD Physics
... • The appropriate boundary conditions to the radial and angular equations leads to the following restrictions on the quantum numbers ℓ and mℓ: – ℓ = 0, 1, 2, 3, . . . – mℓ = −ℓ, −ℓ + 1, . . . , −2, −1, 0, 1, 2, . ℓ . , ℓ − 1, ℓ – |mℓ| ≤ ℓ and ℓ < 0. ...
... • The appropriate boundary conditions to the radial and angular equations leads to the following restrictions on the quantum numbers ℓ and mℓ: – ℓ = 0, 1, 2, 3, . . . – mℓ = −ℓ, −ℓ + 1, . . . , −2, −1, 0, 1, 2, . ℓ . , ℓ − 1, ℓ – |mℓ| ≤ ℓ and ℓ < 0. ...
Electron Configurations
... So you learned about the Bohr model of an atom as well the electronic configuration of that atom. If you have taken or are taking any sort of an advanced chemistry class, then you probably didn’t have much trouble with these concepts. Otherwise, you may want some extra information on the subject. Mo ...
... So you learned about the Bohr model of an atom as well the electronic configuration of that atom. If you have taken or are taking any sort of an advanced chemistry class, then you probably didn’t have much trouble with these concepts. Otherwise, you may want some extra information on the subject. Mo ...
No Slide Title
... the electron is described by a wave function, Y. The exact wavefunction for each electron depends upon four variables, called quantum numbers they are Erwin Schrodinger, an Austrian physicist, proposed that we think of the electrons more as waves than particles. This led to the field called quantum ...
... the electron is described by a wave function, Y. The exact wavefunction for each electron depends upon four variables, called quantum numbers they are Erwin Schrodinger, an Austrian physicist, proposed that we think of the electrons more as waves than particles. This led to the field called quantum ...
Energy levels of various orbitals MEMORIZE ! 1s < 2s < 2p < 3s < 3p
... Energy levels of various orbitals For hydrogen, energy level depend only on n For multielectron atoms (all others) - energy levels depend on both n and l A diagram which shows the orbital energy levels for both is shown below. ...
... Energy levels of various orbitals For hydrogen, energy level depend only on n For multielectron atoms (all others) - energy levels depend on both n and l A diagram which shows the orbital energy levels for both is shown below. ...
Characterizing Atom Sources with Quantum Coherence
... S.S. Hodgman, R.G. Dall, A.G. Manning, M.T. Johnsson, K.G.H. Baldwin ([email protected]. au) and A.G. Truscott are with the Research School of Physics and Engineering, Australian National University, in Canberra, Australia. ...
... S.S. Hodgman, R.G. Dall, A.G. Manning, M.T. Johnsson, K.G.H. Baldwin ([email protected]. au) and A.G. Truscott are with the Research School of Physics and Engineering, Australian National University, in Canberra, Australia. ...
Chemistry 120 Review
... Quantity Relationships in Chemical Reactions • Stoichiometry – Using balanced chemical equations – Calculate the theoretical yield – Calculate percent yield – Limiting Reactant Problems – Thermochemical equations and calculations ...
... Quantity Relationships in Chemical Reactions • Stoichiometry – Using balanced chemical equations – Calculate the theoretical yield – Calculate percent yield – Limiting Reactant Problems – Thermochemical equations and calculations ...
General Chemistry: An Integrated Approach
... ORANGE, do NOT hit metals and get electrons ejected. • However, BLUE, INDIGO, and VIOLET will. • Examples include the solar calculator, papertowel dryers, electronic doors. ...
... ORANGE, do NOT hit metals and get electrons ejected. • However, BLUE, INDIGO, and VIOLET will. • Examples include the solar calculator, papertowel dryers, electronic doors. ...
QUANTUM-MECHANICAL MODEL OF THE ATOM Quantum
... Angular momentum quantum number (l): an integer from 0 to n-1 Indicates the shape of the orbital, sometimes called as “orbital-shape quantum number” n limits l Example: for orbital with n=1 ==> l=0; n=2 ==> l=0,1 Magnetic quantum number (ml): an integer from -1 through 0 to +1 prescribes ...
... Angular momentum quantum number (l): an integer from 0 to n-1 Indicates the shape of the orbital, sometimes called as “orbital-shape quantum number” n limits l Example: for orbital with n=1 ==> l=0; n=2 ==> l=0,1 Magnetic quantum number (ml): an integer from -1 through 0 to +1 prescribes ...
chem 1411- chapter 7
... When energy is given to an atom in the form of heat energy or electrical energy, the electrons in the atom get excited to higher energy levels by absorbing energy. This is the excited state of an atom, which is unstable. The electrons then start falling from higher levels to lower levels, releasing ...
... When energy is given to an atom in the form of heat energy or electrical energy, the electrons in the atom get excited to higher energy levels by absorbing energy. This is the excited state of an atom, which is unstable. The electrons then start falling from higher levels to lower levels, releasing ...