Protein Function
... with the receptor protein, has GDP bound, and has all 3 subunits associated together. • When the membrane receptor binds to its ligand (in this case, the hormone epinephrine), GDP is released and GTP binds. • This leads to the dissociation of the alpha subunit (with GTP) from beta and gamma. ...
... with the receptor protein, has GDP bound, and has all 3 subunits associated together. • When the membrane receptor binds to its ligand (in this case, the hormone epinephrine), GDP is released and GTP binds. • This leads to the dissociation of the alpha subunit (with GTP) from beta and gamma. ...
LS1a Fall 09
... Potassium leak channels selectively allow potassium ions to diffuse down their concentration gradient out of the cell, leaving behind a net negative charge inside the cell and providing the outside of the cell with a slight positive charge. By allowing potassium ions to flow out of the cell, potassi ...
... Potassium leak channels selectively allow potassium ions to diffuse down their concentration gradient out of the cell, leaving behind a net negative charge inside the cell and providing the outside of the cell with a slight positive charge. By allowing potassium ions to flow out of the cell, potassi ...
Midterm 2 review - UCSD Cognitive Science
... What are the roles of G-Proteins, adenylyl cyclase, protein kinases and phosphorylation in metabotropic receptors? ...
... What are the roles of G-Proteins, adenylyl cyclase, protein kinases and phosphorylation in metabotropic receptors? ...
RECEPTORS STRUCTURE AND FUNCTION Chapter 4
... • Many common receptors belong to this same family • Implications for drug selectivity depending on similarity (evolution) • Membrane bound receptors difficult to crystallise • X-Ray structure of bacteriorhodopsin solved - bacterial protein similar to rhodopsin • Bacteriorhodopsin structure used as ...
... • Many common receptors belong to this same family • Implications for drug selectivity depending on similarity (evolution) • Membrane bound receptors difficult to crystallise • X-Ray structure of bacteriorhodopsin solved - bacterial protein similar to rhodopsin • Bacteriorhodopsin structure used as ...
Where in the cell is your protein most likely found?
... observed sequences of proteins of a known function. • TMHMM is a tool used to predict the presence of transmembrane helices in proteins. The results will indicate the segments of the protein that lie inside, outside or within the membrane. ...
... observed sequences of proteins of a known function. • TMHMM is a tool used to predict the presence of transmembrane helices in proteins. The results will indicate the segments of the protein that lie inside, outside or within the membrane. ...
G protein - HCC Learning Web
... Evolution of Cell Signaling • The yeast, Saccharomyces cerevisiae, have two mating types, a and • Cells of different mating types locate each other via secreted factors specific to each type • A signal transduction pathway is a series of steps by which a signal on a cell’s surface is converted in ...
... Evolution of Cell Signaling • The yeast, Saccharomyces cerevisiae, have two mating types, a and • Cells of different mating types locate each other via secreted factors specific to each type • A signal transduction pathway is a series of steps by which a signal on a cell’s surface is converted in ...
RELIATech GmbH
... Description: Produced from sera of rabbits immunised with highly pure recombinant human soluble LYVE-1 produced in insect cells. The recombinant soluble LYVE-1consists of amino acid 24 (Ser) to 232 (Gly) and is fused to a C-terminal His-tag (6xHis). LYVE-1 has been identified as a major receptor for ...
... Description: Produced from sera of rabbits immunised with highly pure recombinant human soluble LYVE-1 produced in insect cells. The recombinant soluble LYVE-1consists of amino acid 24 (Ser) to 232 (Gly) and is fused to a C-terminal His-tag (6xHis). LYVE-1 has been identified as a major receptor for ...
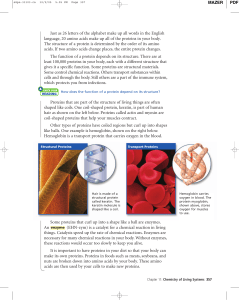
Just as 26 letters of the alphabet make up all words in the English
... language, 20 amino acids make up all of the proteins in your body. The structure of a protein is determined by the order of its amino acids. If two amino acids change places, the entire protein changes. The function of a protein depends on its structure. There are at least 100,000 proteins in your b ...
... language, 20 amino acids make up all of the proteins in your body. The structure of a protein is determined by the order of its amino acids. If two amino acids change places, the entire protein changes. The function of a protein depends on its structure. There are at least 100,000 proteins in your b ...
Alphabodies – working inside the cell
... ability to penetrate through cell membranes, and therefore can only address another 10%, that exist as extracellular proteins. It is therefore estimated that the vast majority of all potential protein targets, more than 80%, are currently considered ‘undruggable’ by the two main classes of therapeut ...
... ability to penetrate through cell membranes, and therefore can only address another 10%, that exist as extracellular proteins. It is therefore estimated that the vast majority of all potential protein targets, more than 80%, are currently considered ‘undruggable’ by the two main classes of therapeut ...
Nanodevices
... Ligand binding to G-protein coupled receptor initiation of cascade-like response ...
... Ligand binding to G-protein coupled receptor initiation of cascade-like response ...
Chapter 5
... 1. Neurotransmitter binds to the receptor protein 2. GDP is released from Ga subunit 3. GTP binds to the a protein cause dissociation of G-protein from the receptor and bg from a subunits 4. Activated a subunit, or the beta-gama complex binds to the effector molecules (signal transduction) 5. GTP wa ...
... 1. Neurotransmitter binds to the receptor protein 2. GDP is released from Ga subunit 3. GTP binds to the a protein cause dissociation of G-protein from the receptor and bg from a subunits 4. Activated a subunit, or the beta-gama complex binds to the effector molecules (signal transduction) 5. GTP wa ...
intro
... Primer on the Olfactory Bulb and Antennal Lobe Brief comparative introduction of: • Neurons • Synaptic interactions ...
... Primer on the Olfactory Bulb and Antennal Lobe Brief comparative introduction of: • Neurons • Synaptic interactions ...
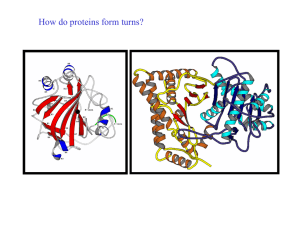
How do proteins form turns? - UF Macromolecular Structure Group
... A reverse turn is region of the polypeptide having a hydrogen bond from one main chain carbonyl oxygen to the main chain N-H group 3 residues along the chain (i.e. O(i) to N(i+3)) Helical regions are excluded from this definition (see later) Reverse turns are very abundant in globular proteins and g ...
... A reverse turn is region of the polypeptide having a hydrogen bond from one main chain carbonyl oxygen to the main chain N-H group 3 residues along the chain (i.e. O(i) to N(i+3)) Helical regions are excluded from this definition (see later) Reverse turns are very abundant in globular proteins and g ...
File - Mrs. LeCompte
... o Hydrophobic side chains orient themselves so that they are minimally exposed to water in the protein’s interior ...
... o Hydrophobic side chains orient themselves so that they are minimally exposed to water in the protein’s interior ...
CH 107 SI Summer 2015 Worksheet 13 Answers What are the two
... 1. What are the two major types of secondary protein structure and what bonds are present in each? α-helices and β-sheets Hydrogen bonds 2. What types of interactions can be present in tertiary protein structure? Rank the interactions from strongest to weakest. disulfide bonds >> salt bridges > hydr ...
... 1. What are the two major types of secondary protein structure and what bonds are present in each? α-helices and β-sheets Hydrogen bonds 2. What types of interactions can be present in tertiary protein structure? Rank the interactions from strongest to weakest. disulfide bonds >> salt bridges > hydr ...
Universal Kinase and GTPase Assays
... NADH is converted to NAD+. This decrease in the concentration of NADH is detected over time based on absorbance. This commonly used kinase detection system is not ideal because it involves three coupled reactions, NADH has a low extinction coefficient, and it measures a decrease in signal rather tha ...
... NADH is converted to NAD+. This decrease in the concentration of NADH is detected over time based on absorbance. This commonly used kinase detection system is not ideal because it involves three coupled reactions, NADH has a low extinction coefficient, and it measures a decrease in signal rather tha ...
The Living World
... One of the three fatty acids is replaced by a phosphate and a small polar functional group ...
... One of the three fatty acids is replaced by a phosphate and a small polar functional group ...
signaling - BrainMass
... 6. Removal of the hormone. Hormones and other signaling molecules may exit the sending cell by exocytosis or other means of membrane transport. The sending cell is typically of a specialized type. Its recipients may be of one type or several, as in the case of insulin, which triggers diverse and sys ...
... 6. Removal of the hormone. Hormones and other signaling molecules may exit the sending cell by exocytosis or other means of membrane transport. The sending cell is typically of a specialized type. Its recipients may be of one type or several, as in the case of insulin, which triggers diverse and sys ...
1. Overview
... • Coordinates can be extracted and viewed • Comparisons of structures allows identification of structural motifs • Proteins with similar functions and sequences = homologs ...
... • Coordinates can be extracted and viewed • Comparisons of structures allows identification of structural motifs • Proteins with similar functions and sequences = homologs ...
Rabbit anti-Estrogen Receptor-β
... Estrogen receptor (ER) is a member of the steroid-receptor family. Unlike protein growth factors that bind to receptors on the cell surface and activate signal-transduction cascades to influence gene expression, the steroid hormones bind to intracellular receptors, which then bind to DNA and regulat ...
... Estrogen receptor (ER) is a member of the steroid-receptor family. Unlike protein growth factors that bind to receptors on the cell surface and activate signal-transduction cascades to influence gene expression, the steroid hormones bind to intracellular receptors, which then bind to DNA and regulat ...
3.2 Proteins - Biology with Radjewski
... • Transport proteins carry substances (e.g., hemoglobin) • Genetic regulatory proteins regulate when, how, and to what extent a gene is expressed ...
... • Transport proteins carry substances (e.g., hemoglobin) • Genetic regulatory proteins regulate when, how, and to what extent a gene is expressed ...
MCB Lecture 3 – ER and Golgi
... o What is the specific mutation called? ∆F508 o How does the mutation affect the protein? It causes the protein to fold incorrectly, so it is ejected out of the ER and degraded in the proteasome. Recent studies have shown that if the protein could make it to the plasma membrane, it could still f ...
... o What is the specific mutation called? ∆F508 o How does the mutation affect the protein? It causes the protein to fold incorrectly, so it is ejected out of the ER and degraded in the proteasome. Recent studies have shown that if the protein could make it to the plasma membrane, it could still f ...
G protein–coupled receptor

G protein–coupled receptors (GPCRs), also known as seven-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptor, and G protein–linked receptors (GPLR), constitute a large protein family of receptors that sense molecules outside the cell and activate inside signal transduction pathways and, ultimately, cellular responses. Coupling with G proteins, they are called seven-transmembrane receptors because they pass through the cell membrane seven times.G protein–coupled receptors are found only in eukaryotes, including yeast, choanoflagellates, and animals. The ligands that bind and activate these receptors include light-sensitive compounds, odors, pheromones, hormones, and neurotransmitters, and vary in size from small molecules to peptides to large proteins. G protein–coupled receptors are involved in many diseases, and are also the target of approximately 40% of all modern medicinal drugs. Two of the United States's top five selling drugs (Hydrocodone and Lisinopril) act by targeting a G protein–coupled receptor. The 2012 Nobel Prize in Chemistry was awarded to Brian Kobilka and Robert Lefkowitz for their work that was ""crucial for understanding how G protein–coupled receptors function."". There have been at least seven other Nobel Prizes awarded for some aspect of G protein–mediated signaling.There are two principal signal transduction pathways involving the G protein–coupled receptors: the cAMP signal pathway and the phosphatidylinositol signal pathway. When a ligand binds to the GPCR it causes a conformational change in the GPCR, which allows it to act as a guanine nucleotide exchange factor (GEF). The GPCR can then activate an associated G protein by exchanging its bound GDP for a GTP. The G protein's α subunit, together with the bound GTP, can then dissociate from the β and γ subunits to further affect intracellular signaling proteins or target functional proteins directly depending on the α subunit type (Gαs, Gαi/o, Gαq/11, Gα12/13).