CPphysics review 2-10
... 20) A diver with a mass of 80.0 kg jumps from a dock into a 130.0 kg boat at rest on the west side of the dock. If the velocity of the diver in the air is 4.10 m/s to the west, what is the final velocity of the diver after landing in the boat (diver and boat move with same ...
... 20) A diver with a mass of 80.0 kg jumps from a dock into a 130.0 kg boat at rest on the west side of the dock. If the velocity of the diver in the air is 4.10 m/s to the west, what is the final velocity of the diver after landing in the boat (diver and boat move with same ...
• Quantum physics explains the energy levels of atoms with
... • Quantum physics explains the energy levels of atoms with enormous accuracy. This is possible, since these levels have long lifetime (uncertainty relation for E, t). • Radiation from atoms and molecules enables the most accurate time and length measurements: Atomic clocks • Quantum physics explai ...
... • Quantum physics explains the energy levels of atoms with enormous accuracy. This is possible, since these levels have long lifetime (uncertainty relation for E, t). • Radiation from atoms and molecules enables the most accurate time and length measurements: Atomic clocks • Quantum physics explai ...
a previous Learning Experience
... A particle of mass m = 2.00 kg has a velocity of v = 4.00 m/sec parallel to the x-axis. What is the angular momentum of the particle about the origin when its position is at r = 2.00 m from the origin along a line making a 30o angle with the x-axis? [Including direction!] ...
... A particle of mass m = 2.00 kg has a velocity of v = 4.00 m/sec parallel to the x-axis. What is the angular momentum of the particle about the origin when its position is at r = 2.00 m from the origin along a line making a 30o angle with the x-axis? [Including direction!] ...
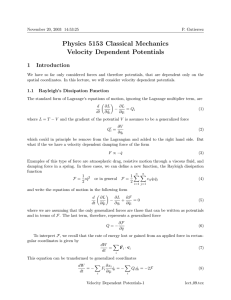
Physics 5153 Classical Mechanics Velocity Dependent Potentials
... so if any of the coordinates are cyclic, it is this momentum that is conserved. That is a portion of the momentum of the particle is associated with the electromagnetic field. The energy of the particle is E = T + eφ ...
... so if any of the coordinates are cyclic, it is this momentum that is conserved. That is a portion of the momentum of the particle is associated with the electromagnetic field. The energy of the particle is E = T + eφ ...
SCI24TutJan15th
... A transport truck with a mass of 10 000 kg and a car with a mass of 2000 kg are travelling at the same velocity (100 km/h) but in opposite directions. The truck is travelling to the left, and has a momentum of – 1 000 000 kg.km/h. The car is moving to the right, and has a momentum of +200 000 kg.km ...
... A transport truck with a mass of 10 000 kg and a car with a mass of 2000 kg are travelling at the same velocity (100 km/h) but in opposite directions. The truck is travelling to the left, and has a momentum of – 1 000 000 kg.km/h. The car is moving to the right, and has a momentum of +200 000 kg.km ...
Study Guide: Chapter 4 - the Arrangement of Electrons in Atoms
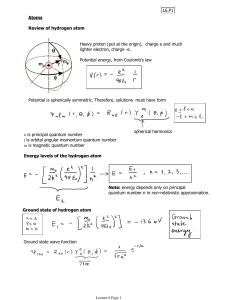
... 8. Understand the 4 quantum numbers: a. The principle quantum number (n) = main energy level; indicates the energy of an electron and the distance from the nucleus; n2 = total number of orbitals in that main energy level; 2n2 = total number of electrons in that main energy level b. The angular momen ...
... 8. Understand the 4 quantum numbers: a. The principle quantum number (n) = main energy level; indicates the energy of an electron and the distance from the nucleus; n2 = total number of orbitals in that main energy level; 2n2 = total number of electrons in that main energy level b. The angular momen ...
rest energy - Purdue Physics
... believed to be satisfied by all the laws of physics, including the theory of special relativity • The momentum of a single particle can also be written as Dx p = m0 Dt Section 27.7 ...
... believed to be satisfied by all the laws of physics, including the theory of special relativity • The momentum of a single particle can also be written as Dx p = m0 Dt Section 27.7 ...
Slide 101
... 4. Consider a system of particles that are indistinguishable but for the purposes of constructing wavefunctions can be numbered from 1 to N. These particles are simultaneously confined in some potential. Each of them could be in any energy state from the selection {a, b, c, ... n}. If any one of the ...
... 4. Consider a system of particles that are indistinguishable but for the purposes of constructing wavefunctions can be numbered from 1 to N. These particles are simultaneously confined in some potential. Each of them could be in any energy state from the selection {a, b, c, ... n}. If any one of the ...
0321813545_07_final
... Know that electrons and photons behave in similar ways: both can act as particles and as waves. Know that photons and electrons, even when viewed as streams of particles, still display diffraction and interference patterns in a double‐slit experiment. Use de Broglie’s relation to interconvert wa ...
... Know that electrons and photons behave in similar ways: both can act as particles and as waves. Know that photons and electrons, even when viewed as streams of particles, still display diffraction and interference patterns in a double‐slit experiment. Use de Broglie’s relation to interconvert wa ...
Chapter 30 Quantum Physics
... In 1923, de Broglie proposed that, as waves can exhibit particle-like behavior, particles should exhibit wave-like behavior as well. He proposed that the same relationship between wavelength and momentum should apply to massive particles as well as photons: ...
... In 1923, de Broglie proposed that, as waves can exhibit particle-like behavior, particles should exhibit wave-like behavior as well. He proposed that the same relationship between wavelength and momentum should apply to massive particles as well as photons: ...
N/Z = 2, 8, 20, 28, 50, 82, 126
... then the eigenstates have definite orbital angular momentum, and the standard radial and angular momentum quantum numbers (n,l,m) as indicated. (Justification: measured quadrupole moments of nuclei are relatively small, at least near the “magic numbers” that we are interested in explaining; midway b ...
... then the eigenstates have definite orbital angular momentum, and the standard radial and angular momentum quantum numbers (n,l,m) as indicated. (Justification: measured quadrupole moments of nuclei are relatively small, at least near the “magic numbers” that we are interested in explaining; midway b ...
CH160: Professor Peter Sadler Introduction to inorganic chemistry
... - Rutherford concluded that atoms contain a verysmall (compared with size of the atom) positive charge, which can repel alpha particles if comes close enough 1911 Rutherford model of atom Electrons move in circular orbits around positively-charged nucleus - classical picture, electrons obey Newton’s ...
... - Rutherford concluded that atoms contain a verysmall (compared with size of the atom) positive charge, which can repel alpha particles if comes close enough 1911 Rutherford model of atom Electrons move in circular orbits around positively-charged nucleus - classical picture, electrons obey Newton’s ...
phys3313-fall13
... • In quantum mechanics, measurements can only be expressed in terms of average behaviors since precision measurement of each event is impossible (what principle is this?) • The expectation value is the expected result of the average of many measurements of a given quantity. The expectation value of ...
... • In quantum mechanics, measurements can only be expressed in terms of average behaviors since precision measurement of each event is impossible (what principle is this?) • The expectation value is the expected result of the average of many measurements of a given quantity. The expectation value of ...
Classical Ideal Gas
... as given for example by Eq. (21.11) of Stowe, where E is the total internal energy of the gas. Invoking the equipartition theorem as ...
... as given for example by Eq. (21.11) of Stowe, where E is the total internal energy of the gas. Invoking the equipartition theorem as ...