Intermolecular forces and molecules
... boiling point. Do electronegativity differences account for this difference? Due to the molecule geometry, the bond dipoles in each cancel. In this case, differences in molar mass (polarizability) account for the variation in boiling point. Having noted that molar mass affects van der Waals forces, ...
... boiling point. Do electronegativity differences account for this difference? Due to the molecule geometry, the bond dipoles in each cancel. In this case, differences in molar mass (polarizability) account for the variation in boiling point. Having noted that molar mass affects van der Waals forces, ...
Module 2 Alcohols, halogenoalkanes and analysis
... Alcoh and analysis Introduction Throughout the centuries, chemists have synthesised new substances and investigated their properties in the search for more useful materials. In the recent past, organic chemists have developed a broad range of original and exciting materials, such as pharmaceuticals, ...
... Alcoh and analysis Introduction Throughout the centuries, chemists have synthesised new substances and investigated their properties in the search for more useful materials. In the recent past, organic chemists have developed a broad range of original and exciting materials, such as pharmaceuticals, ...
Chemistry 30 – Organic Chemistry
... moleculars – temporary dipoles – affected by total # of e- and shape • Dipole-dipole Forces – polar moleculars • Hydrogen Bonding (H covalently bonded to F, O, or N) ...
... moleculars – temporary dipoles – affected by total # of e- and shape • Dipole-dipole Forces – polar moleculars • Hydrogen Bonding (H covalently bonded to F, O, or N) ...
Chemistry
... particle of matter. It translates to mean something that is indivisible. In the eighteenth century, chemist, John Dalton, revived the term when he suggested that each element was made up of unique atoms and the atoms of an element are all the same. At that time, there were about 35 known elements. T ...
... particle of matter. It translates to mean something that is indivisible. In the eighteenth century, chemist, John Dalton, revived the term when he suggested that each element was made up of unique atoms and the atoms of an element are all the same. At that time, there were about 35 known elements. T ...
pretest - Allen County Schools
... 6. Identify water condensing on the outside of a glass of ice water. a. physical change b. chemical change 7. You can cause water to change state of matter by removing or adding what? a. matter c. energy b. particles d. surface tension 8. The temperature at which a substance changes from a solid to ...
... 6. Identify water condensing on the outside of a glass of ice water. a. physical change b. chemical change 7. You can cause water to change state of matter by removing or adding what? a. matter c. energy b. particles d. surface tension 8. The temperature at which a substance changes from a solid to ...
Chapter 1 Chemistry: The Study of Matter
... Chromatography is a method of separating mixtures that uses a stationary phase and a mobile phase. Paper chromatography can be used to separate pigments because they move at different rates on the paper. ...
... Chromatography is a method of separating mixtures that uses a stationary phase and a mobile phase. Paper chromatography can be used to separate pigments because they move at different rates on the paper. ...
Honors Mid-Term Review Sheet
... 27. What is the mass of 180.3 cm3 of lead if the density is 11.4 g/cm3? 28. What is the density of 325g of a substance with a volume of 492mL? 29. Define accuracy and precision. 30. Complete the following calculations with the correct number of significant figures: a. 1.23kg + 4.082kg b. 16.04s – 5 ...
... 27. What is the mass of 180.3 cm3 of lead if the density is 11.4 g/cm3? 28. What is the density of 325g of a substance with a volume of 492mL? 29. Define accuracy and precision. 30. Complete the following calculations with the correct number of significant figures: a. 1.23kg + 4.082kg b. 16.04s – 5 ...
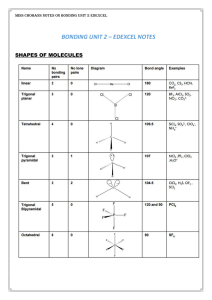
Unit 3: Bonding and Nomenclature Content Outline: Chemical
... A. These molecules are composed of positive and negative ions that are combined in a lattice (3D cube) like structure that looks “like” a crystal (crystalline). 1. The ions alternate (positive- negative) so as to maintain neutrality and reduce repulsive forces between like charged ions. 2. The attra ...
... A. These molecules are composed of positive and negative ions that are combined in a lattice (3D cube) like structure that looks “like” a crystal (crystalline). 1. The ions alternate (positive- negative) so as to maintain neutrality and reduce repulsive forces between like charged ions. 2. The attra ...
Origin of Order: Emergence and Evolution of Biological Organization
... between heat and other forms of energy. Thermodynamics is also concerned with systems of very large numbers of particles so that thermodynamic variables, such as pressure, volume, and temperature, are considered as statistical quantities. This field of science is concerned with the changes of energy ...
... between heat and other forms of energy. Thermodynamics is also concerned with systems of very large numbers of particles so that thermodynamic variables, such as pressure, volume, and temperature, are considered as statistical quantities. This field of science is concerned with the changes of energy ...
Instructor notes
... The reaction also occurs without retention of configuration, making it unlikely that the products of such a reaction would be usefully related to the reactants. Metal complexes can participate in the reaction, but only in the context of propagating the radical chain. The oxenoid mechanism is much mo ...
... The reaction also occurs without retention of configuration, making it unlikely that the products of such a reaction would be usefully related to the reactants. Metal complexes can participate in the reaction, but only in the context of propagating the radical chain. The oxenoid mechanism is much mo ...
Chemistry Honours - SCS Autonomous College
... Intensive and extensive variables; state and path functions; isolated, closed and open systems; zeroth law of thermodynamics. First law: Concept of heat, q, work, w, internal energy, U, and statement of first law; enthalpy, H, relation between heat capacities, calculations of q, w, U and H for rever ...
... Intensive and extensive variables; state and path functions; isolated, closed and open systems; zeroth law of thermodynamics. First law: Concept of heat, q, work, w, internal energy, U, and statement of first law; enthalpy, H, relation between heat capacities, calculations of q, w, U and H for rever ...
Lecture 6
... More selective towards the formation of a single product More active More easilyy studied to understand the chemical and mechanistic aspects p More easily modifiable for optimizing selectivity ...
... More selective towards the formation of a single product More active More easilyy studied to understand the chemical and mechanistic aspects p More easily modifiable for optimizing selectivity ...
functional groups 2. PPT
... C=O is polar & their compounds Are polar. This leads to: -Higher B.P’s than non-polar molecules -Lower than alcohols that can H-bond w/ each other -Can not H-bond between each other but can H-bond to solvents like water - Lower MW compounds are appreciably soluble in water ...
... C=O is polar & their compounds Are polar. This leads to: -Higher B.P’s than non-polar molecules -Lower than alcohols that can H-bond w/ each other -Can not H-bond between each other but can H-bond to solvents like water - Lower MW compounds are appreciably soluble in water ...
File
... form. This makes the London forces stronger between the molecules and more energy is needed to break them so boiling points will be greater The increasing boiling points of the halogens down the group 7 series can be explained by the increasing number of electrons in the bigger molecules causing a ...
... form. This makes the London forces stronger between the molecules and more energy is needed to break them so boiling points will be greater The increasing boiling points of the halogens down the group 7 series can be explained by the increasing number of electrons in the bigger molecules causing a ...
Compounds and molecules: - Wikispaces
... electron while Chlorine atom gains that electron to complete its octet-system of 8-electrons. As a result, NaCl comp. is formed. • Sodium atom becomes positive ion by losing electron and Chlorine atom becomes negative ion by gaining electron. Therefore Na+ and Cl- combine forming ionic bond between ...
... electron while Chlorine atom gains that electron to complete its octet-system of 8-electrons. As a result, NaCl comp. is formed. • Sodium atom becomes positive ion by losing electron and Chlorine atom becomes negative ion by gaining electron. Therefore Na+ and Cl- combine forming ionic bond between ...