A New Discontinuous Petrov-Galerkin Method with Optimal Test
... For nonlinear shock problems, the solution often exhibits sharp gradients or discontinuities, around which the solution would develop spurious Gibbs-type oscillations. Several ideas were introduced to deal with oscillations in the solution near a sharp gradient or shock: artificial viscosity paramet ...
... For nonlinear shock problems, the solution often exhibits sharp gradients or discontinuities, around which the solution would develop spurious Gibbs-type oscillations. Several ideas were introduced to deal with oscillations in the solution near a sharp gradient or shock: artificial viscosity paramet ...
4 Unit Packet - SRHSchem
... 3. Is it possible, given the original data in Table 1, to determine the % composition by mass of H for 2-butene without using the equation given in the model? If so, how? ...
... 3. Is it possible, given the original data in Table 1, to determine the % composition by mass of H for 2-butene without using the equation given in the model? If so, how? ...
physical setting chemistry
... this examination according to the directions provided in the examination booklet. Your answer sheet for Part A and Part B–1 is the last page of this examination booklet. Turn to the last page and fold it along the perforations. Then, slowly and carefully, tear off your answer sheet and fill in the h ...
... this examination according to the directions provided in the examination booklet. Your answer sheet for Part A and Part B–1 is the last page of this examination booklet. Turn to the last page and fold it along the perforations. Then, slowly and carefully, tear off your answer sheet and fill in the h ...
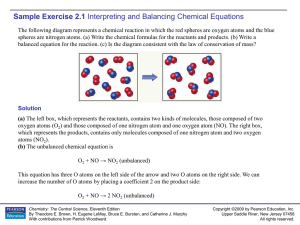
Sample Exercise 2.1
... When any compound containing C, H, and O is combusted, it reacts with the O 2(g) in air to produce CO2(g) and H2O(g). Thus, the unbalanced equation is CH3OH(l) + O2(g) → CO2(g) + H2O(g) In this equation the C atoms are balanced with one carbon on each side of the arrow. Because CH 3OH has four H ato ...
... When any compound containing C, H, and O is combusted, it reacts with the O 2(g) in air to produce CO2(g) and H2O(g). Thus, the unbalanced equation is CH3OH(l) + O2(g) → CO2(g) + H2O(g) In this equation the C atoms are balanced with one carbon on each side of the arrow. Because CH 3OH has four H ato ...
Chemistry
... 440 BC, the Greek philosopher Leucippus and his pupil Democritus coined the term atomos to describe the smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth century, the chemist John Dalton, revived the term when he suggested that each element was made ...
... 440 BC, the Greek philosopher Leucippus and his pupil Democritus coined the term atomos to describe the smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth century, the chemist John Dalton, revived the term when he suggested that each element was made ...
Ch.1-Matter and Change
... In the liquid state, matter has a definite volume, but an indefinite shape. In the gaseous state, matter has neither definite volume nor definite shape. Plasma is a high-temperature physical state of matter in which atoms lose most of their electrons, particles that make up atoms. ...
... In the liquid state, matter has a definite volume, but an indefinite shape. In the gaseous state, matter has neither definite volume nor definite shape. Plasma is a high-temperature physical state of matter in which atoms lose most of their electrons, particles that make up atoms. ...
Manual Physical Chemistry III
... molecules (on the order of 0.1 nm). Thus the compressibility of liquids is lower than that of gas, while the density is much higher. On the other hand, these cohesive forces are not strong enough to result into the fixed position of molecules that can be seen in solid matter. Liquids do not keep a f ...
... molecules (on the order of 0.1 nm). Thus the compressibility of liquids is lower than that of gas, while the density is much higher. On the other hand, these cohesive forces are not strong enough to result into the fixed position of molecules that can be seen in solid matter. Liquids do not keep a f ...
Enhancing the secondary-tertiary transition in
... a new world that is very different to their high-school environment. At many Australian tertiary institutions, incoming students experience very large lecture theatres with classes of hundreds of students, and the delivery and assessment of content is markedly different to high school. From the inst ...
... a new world that is very different to their high-school environment. At many Australian tertiary institutions, incoming students experience very large lecture theatres with classes of hundreds of students, and the delivery and assessment of content is markedly different to high school. From the inst ...
Chapter 3 Stoichiometry: Calculations with Chemical
... (a). the same as the percent by mass weight (b). determined by combustion analysis (c). the sum of atomic weights of each atom in its chemical formula (d). the weight of a sample of the substance. 33. The mass % of C in methane (CH4) is _________. (a). 25.13 (b). 13.36 (c). 92.26 (d).74.87 Explanati ...
... (a). the same as the percent by mass weight (b). determined by combustion analysis (c). the sum of atomic weights of each atom in its chemical formula (d). the weight of a sample of the substance. 33. The mass % of C in methane (CH4) is _________. (a). 25.13 (b). 13.36 (c). 92.26 (d).74.87 Explanati ...
Chapter 3 PowerPoint
... What is the actual yield? What is the theoretical yield? What is the percent yield? If you had started with 9.73 g of Al, how ...
... What is the actual yield? What is the theoretical yield? What is the percent yield? If you had started with 9.73 g of Al, how ...
Challenge Problems
... he chemical properties of an element depend primarily on its number of valence electrons in its atoms. The noble gas elements, for example, all have similar chemical properties because the outermost energy levels of their atoms are completely filled. The chemical properties of ions also depend on th ...
... he chemical properties of an element depend primarily on its number of valence electrons in its atoms. The noble gas elements, for example, all have similar chemical properties because the outermost energy levels of their atoms are completely filled. The chemical properties of ions also depend on th ...