The Discriminant
... (roots) you will get. The discriminant can be +, –, or 0 which actually tells you a lot! Since the discriminant is under a radical, think about what it means if you have a positive or negative number or 0 under the radical. ...
... (roots) you will get. The discriminant can be +, –, or 0 which actually tells you a lot! Since the discriminant is under a radical, think about what it means if you have a positive or negative number or 0 under the radical. ...
solving for a variable
... Solving for a Variable Example 2B: Solving Formulas for a Variable The formula for a person’s typing speed is ,where s is speed in words per minute, w is number of words typed, e is number of errors, and m is number of minutes typing. Solve for e. Locate e in the equation. Since w–10e is divided by ...
... Solving for a Variable Example 2B: Solving Formulas for a Variable The formula for a person’s typing speed is ,where s is speed in words per minute, w is number of words typed, e is number of errors, and m is number of minutes typing. Solve for e. Locate e in the equation. Since w–10e is divided by ...
No Slide Title
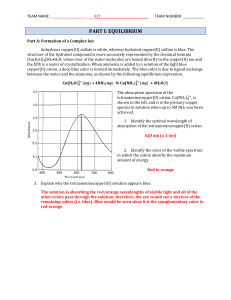
... How would you prepare 60.0 mL of 0.200 M HNO3 from a stock solution of 4.00 M HNO3? ...
... How would you prepare 60.0 mL of 0.200 M HNO3 from a stock solution of 4.00 M HNO3? ...
Advanced Placement Chemistry
... (D) Flush the affected area with water and then with a dilute NaHCO3 solution (E) Flush the affected area with water and then with a dilute vinegar solution 25. The cooling curve for a pure substance as it changes from a liquid to a solid is shown right. The solid and the liquid coexist at (A) point ...
... (D) Flush the affected area with water and then with a dilute NaHCO3 solution (E) Flush the affected area with water and then with a dilute vinegar solution 25. The cooling curve for a pure substance as it changes from a liquid to a solid is shown right. The solid and the liquid coexist at (A) point ...
1999 Advanced Placement Chemistry Exam Section I: Multiple
... 42. When the equation above is balanced and all co(A) A dark red precipitate forms and settles out. efficients reduced to lowest whole-number (B) Separate layers of immiscible liquids form terms, the coefficient for OH–(aq) is with a blue layer on top. (A) 1 (B) 2 (C) 3 (C) The color of the solution ...
... 42. When the equation above is balanced and all co(A) A dark red precipitate forms and settles out. efficients reduced to lowest whole-number (B) Separate layers of immiscible liquids form terms, the coefficient for OH–(aq) is with a blue layer on top. (A) 1 (B) 2 (C) 3 (C) The color of the solution ...
Equations and Mental Math
... To answer the question above about backpacking, you can use the problem solving strategy guess, check, and revise. 1 Try an item on the list. ...
... To answer the question above about backpacking, you can use the problem solving strategy guess, check, and revise. 1 Try an item on the list. ...
Ch. 5 Review Guide
... Brenda's school is selling tickets to a spring musical. On the first day of ticket sales the school sold 3 senior citizen tickets and 9 child tickets for a total of $75. The school took in $67 on the second day by selling 8 senior citizen tickets and 5 child tickets. What is the price each of one se ...
... Brenda's school is selling tickets to a spring musical. On the first day of ticket sales the school sold 3 senior citizen tickets and 9 child tickets for a total of $75. The school took in $67 on the second day by selling 8 senior citizen tickets and 5 child tickets. What is the price each of one se ...
system of equations - Gordon State College
... A solution of a system of equations consists of values for the variables that are solutions of each equation in the system. To solve a system of equations means to find all solutions to the system. ...
... A solution of a system of equations consists of values for the variables that are solutions of each equation in the system. To solve a system of equations means to find all solutions to the system. ...
111 Exam I Outline
... 6) 3.9104 g sample of a compound made of carbon, hydrogen, nitrogen, and oxygen is burned completely. 3.820 g CO2 and 3.125 g H2O are produced. Analysis of nitrogen showed that the compound contains 46.62 % by mass nitrogen. The molar mass of the compound is about 170 + 15 g/mole. a) Calculate the e ...
... 6) 3.9104 g sample of a compound made of carbon, hydrogen, nitrogen, and oxygen is burned completely. 3.820 g CO2 and 3.125 g H2O are produced. Analysis of nitrogen showed that the compound contains 46.62 % by mass nitrogen. The molar mass of the compound is about 170 + 15 g/mole. a) Calculate the e ...
GCE Chemistry Question Paper Unit 05 - Energetics, Redox
... Use your equation from part (e) to explain why the free-energy change for the reaction to form nitrogen monoxide stays approximately constant at different temperatures. ...
... Use your equation from part (e) to explain why the free-energy change for the reaction to form nitrogen monoxide stays approximately constant at different temperatures. ...
Name - Rochester Community Schools
... 17. A landscape supply business charges $30 to deliver mulch. The mulch costs $23 per cubic yard. a. Write an equation that gives the total cost C of having mulch m delivered to a site as a function of the number of cubic yards ordered. ...
... 17. A landscape supply business charges $30 to deliver mulch. The mulch costs $23 per cubic yard. a. Write an equation that gives the total cost C of having mulch m delivered to a site as a function of the number of cubic yards ordered. ...
cbse class – x science solutions
... found to be the highest. Name and state the rule used to known the direction of the induced current. (i) ...
... found to be the highest. Name and state the rule used to known the direction of the induced current. (i) ...
Part II - American Chemical Society
... a score on Part II. Testing materials, scratch paper, and the “Blue Book” should be made available to the student only during the examination period. All testing materials including scratch paper should be turned in and kept secure until April 27, 2005, after which tests can be returned to students ...
... a score on Part II. Testing materials, scratch paper, and the “Blue Book” should be made available to the student only during the examination period. All testing materials including scratch paper should be turned in and kept secure until April 27, 2005, after which tests can be returned to students ...