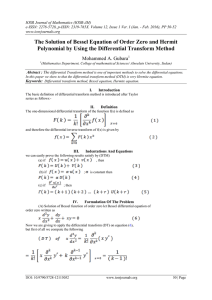
Chapter 2
... Solving Equations Example: Solve the equation 3(4 – x) + 5x = 9. 12 – 3x + 5x = 9 (Distributive property) 12 + 2x = 9 (Combine like terms.) 12 - 12 + 2x = 9 - 12 (Add –12 to both sides.) 2x = -3 (Divide both sides by 2.) x 3 ...
... Solving Equations Example: Solve the equation 3(4 – x) + 5x = 9. 12 – 3x + 5x = 9 (Distributive property) 12 + 2x = 9 (Combine like terms.) 12 - 12 + 2x = 9 - 12 (Add –12 to both sides.) 2x = -3 (Divide both sides by 2.) x 3 ...
Unit #7 Take Home Test
... 36. An unsaturated solution is made by completely dissolving 20.0 grams of NaNO3 in 100.0 grams of water at 20.0°C. Calculate the number of moles of NaNO3 used to make this unsaturated solution. [2] ...
... 36. An unsaturated solution is made by completely dissolving 20.0 grams of NaNO3 in 100.0 grams of water at 20.0°C. Calculate the number of moles of NaNO3 used to make this unsaturated solution. [2] ...
Ch 8 Notes: Chemical Equations and Reactions
... Rules for Predicting Double Replacement Reactions: 1. Predict the products of the double-replacement reaction and indicate the solubility of both of the products by placing the symbol "(aq)" after the soluble product and the symbol "(s)" after the insoluble product. Use the “Solubility Rules” handou ...
... Rules for Predicting Double Replacement Reactions: 1. Predict the products of the double-replacement reaction and indicate the solubility of both of the products by placing the symbol "(aq)" after the soluble product and the symbol "(s)" after the insoluble product. Use the “Solubility Rules” handou ...
Chapter 8
... crystal lattice Lattice energy is a result of the electrostatic attractions between oppositely charged ions There is an inverse relationship between lattice energy and interionic distance ...
... crystal lattice Lattice energy is a result of the electrostatic attractions between oppositely charged ions There is an inverse relationship between lattice energy and interionic distance ...
converting a repeating decimal to a fraction
... that appear under the bar. 3. If applicable, rewrite the second equation so that its repeating part lines up with the repeating part in the original equation. 4. Subtract the original equation from the most recently obtained equation. (The repeating part should cancel at this step.) 5. If applicable ...
... that appear under the bar. 3. If applicable, rewrite the second equation so that its repeating part lines up with the repeating part in the original equation. 4. Subtract the original equation from the most recently obtained equation. (The repeating part should cancel at this step.) 5. If applicable ...
For best results please view this as a slide show. You can hit the F5
... Description of Empirical Formula An empirical formula gives the relative numbers of atoms of each element present in a chemical compound. For example, the formula NaCl, indicates that in this compound there is one Na atom for every Cl. In H2O there are two hydrogen atoms for every oxygen atom. By n ...
... Description of Empirical Formula An empirical formula gives the relative numbers of atoms of each element present in a chemical compound. For example, the formula NaCl, indicates that in this compound there is one Na atom for every Cl. In H2O there are two hydrogen atoms for every oxygen atom. By n ...
Learning Outcomes for Chemical Reactions and
... • Identify whether a species has an equal or unequal number of protons and electrons and use this to state whether it is an atom or ion. • State the charge of an ion. • Calculate the charge on a ion using nuclide notation • Use the periodic table to identify whether an element is a metal or non-meta ...
... • Identify whether a species has an equal or unequal number of protons and electrons and use this to state whether it is an atom or ion. • State the charge of an ion. • Calculate the charge on a ion using nuclide notation • Use the periodic table to identify whether an element is a metal or non-meta ...
ppt Sc10 Review Notes
... eg) Li(s), Cu(s), Hg(l) nonmetals and hydrogen do not exist as single atoms – flagpole! ...
... eg) Li(s), Cu(s), Hg(l) nonmetals and hydrogen do not exist as single atoms – flagpole! ...
unit 2 review
... Give the percentage composition for each compound: a) H2SO4, b) Ca(OH)2. Mg has 3 isotopes: 24Mg(78.7%), 25Mg(10.1%), 26Mg(11.2%). Give the average atomic mass. Calculate the molar mass of a) H2SO4, b) Fe2(Cr2O7)3. a) How many moles are in 16 grams of CuCl2? b) How much does 70 moles of NaCl weigh? ...
... Give the percentage composition for each compound: a) H2SO4, b) Ca(OH)2. Mg has 3 isotopes: 24Mg(78.7%), 25Mg(10.1%), 26Mg(11.2%). Give the average atomic mass. Calculate the molar mass of a) H2SO4, b) Fe2(Cr2O7)3. a) How many moles are in 16 grams of CuCl2? b) How much does 70 moles of NaCl weigh? ...
7-5 - Ithaca Public Schools
... An extraneous solution satisfies later equations in your work but does not make the original equation true. All rights reserved. ...
... An extraneous solution satisfies later equations in your work but does not make the original equation true. All rights reserved. ...