Sensors Technology MED4: Electron Flow – Current
... As each electron moves uniformly through a conductor, it pushes on the one ahead of it, such that all the electrons move together as a group. The starting and stopping of electron flow through the length of a conductive path is virtually instantaneous from one end of a conductor to the other, even t ...
... As each electron moves uniformly through a conductor, it pushes on the one ahead of it, such that all the electrons move together as a group. The starting and stopping of electron flow through the length of a conductive path is virtually instantaneous from one end of a conductor to the other, even t ...
SM72295 Photovoltaic Full Bridge Driver (Rev. E)
... considerations during circuit board layout. Following points are emphasized. 1. Low ESR / ESL capacitors must be connected close to the IC, between VDD and VSS pins and between the HB and HS pins to support the high peak currents being drawn from VDD during turn-on of the external MOSFET. 2. To prev ...
... considerations during circuit board layout. Following points are emphasized. 1. Low ESR / ESL capacitors must be connected close to the IC, between VDD and VSS pins and between the HB and HS pins to support the high peak currents being drawn from VDD during turn-on of the external MOSFET. 2. To prev ...
AP Chemistry Review Preparing for the AP
... Give examples and solve calculation problems related to each of the three theories. Sketch a cathode ray tube as demonstrated in class and state how J.J. Thomson’s experiments led to the idea that atoms have positive and negative parts, the negative parts are all the same, and the negative parts ...
... Give examples and solve calculation problems related to each of the three theories. Sketch a cathode ray tube as demonstrated in class and state how J.J. Thomson’s experiments led to the idea that atoms have positive and negative parts, the negative parts are all the same, and the negative parts ...
投影片 1 - CUST
... • For Ps can be approximated by 4 . • An important observation is that Ps decreases only inversely with the average received SNR . • On the other hand, when there is no fading, Ps decreases exponentially with the received SNR (which is a constant). • Therefore, a much larger amount of energy is req ...
... • For Ps can be approximated by 4 . • An important observation is that Ps decreases only inversely with the average received SNR . • On the other hand, when there is no fading, Ps decreases exponentially with the received SNR (which is a constant). • Therefore, a much larger amount of energy is req ...
AP Chemistry: Course Introduction Sheet
... They go through several examples of the types of problems I have assigned. If you cannot find my webpage, email me and I will send you the link. •AP Chemistry Boot Camp: You are highly encouraged to sign up for AP Chemistry Boot Camp. We will mostly be covering Units 3 and 4 during boot camp, but if ...
... They go through several examples of the types of problems I have assigned. If you cannot find my webpage, email me and I will send you the link. •AP Chemistry Boot Camp: You are highly encouraged to sign up for AP Chemistry Boot Camp. We will mostly be covering Units 3 and 4 during boot camp, but if ...
Sec 5.8 - 5.11 notes
... Example; A student has 3 metals: Ag, Zn and Cu; three solutions: AgNO3, Zn(NO3)2, and Cu(NO3)2, all 1M. She also has a salt bridge containing KNO3 (aq) wires and a voltmeter. a) Which combination of 2 metals and 2 solutions should she choose to get the highest possible voltage? ...
... Example; A student has 3 metals: Ag, Zn and Cu; three solutions: AgNO3, Zn(NO3)2, and Cu(NO3)2, all 1M. She also has a salt bridge containing KNO3 (aq) wires and a voltmeter. a) Which combination of 2 metals and 2 solutions should she choose to get the highest possible voltage? ...
Pulse Testing for Nanoscale Devices
... after a transistor is turned on. As charges are trapped in the gate dielectric, the threshold ...
... after a transistor is turned on. As charges are trapped in the gate dielectric, the threshold ...
1 Electrician`s Math and Basic Electrical Formulas
... Every student begins at a different level of understanding, and you may find this unit an easy review, or you may find it requires a high level of concentration. In any case, be certain that you fully understand the concepts of this unit and are able to successfully complete the questions at the end ...
... Every student begins at a different level of understanding, and you may find this unit an easy review, or you may find it requires a high level of concentration. In any case, be certain that you fully understand the concepts of this unit and are able to successfully complete the questions at the end ...
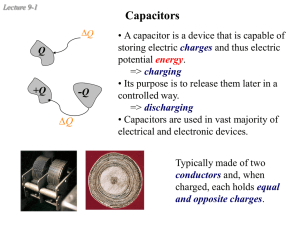
+Q - Purdue Physics
... A capacitor C0 with air dielectric κ =1 is charged to a potential V and then is disconnected from the battery. A uniform dielectric with dielectric constant κ > 1 is inserted into the capacitor and occupies the full volume of the capacitor. If the original electric field is E0 and the final electric ...
... A capacitor C0 with air dielectric κ =1 is charged to a potential V and then is disconnected from the battery. A uniform dielectric with dielectric constant κ > 1 is inserted into the capacitor and occupies the full volume of the capacitor. If the original electric field is E0 and the final electric ...
Document
... Calculate the molecular formula of quinine, whose molecular weight is 324.41 g/mol. Analysis of quinine gives 74.04 % C, 7.46% H, 9.86 % O, and 8.63% N. ...
... Calculate the molecular formula of quinine, whose molecular weight is 324.41 g/mol. Analysis of quinine gives 74.04 % C, 7.46% H, 9.86 % O, and 8.63% N. ...
Glossary: Chemical bonds
... of isotopic masses found in a typical terrestrial sample of the element. Atom. Compare with molecule and ion. An atom is the smallest particle of an element that retains the chemical properties of the element. Atoms are electrically neutral, with a positively charged nucleus that binds one or more e ...
... of isotopic masses found in a typical terrestrial sample of the element. Atom. Compare with molecule and ion. An atom is the smallest particle of an element that retains the chemical properties of the element. Atoms are electrically neutral, with a positively charged nucleus that binds one or more e ...
lecture1427131830
... This document does not claim any originality and cannot be used as a substitute for prescribed textbooks. The information presented here is merely a collection by the committee members for their respective teaching assignments. Various sources of the document as well as freely available material fro ...
... This document does not claim any originality and cannot be used as a substitute for prescribed textbooks. The information presented here is merely a collection by the committee members for their respective teaching assignments. Various sources of the document as well as freely available material fro ...
analytical chemistry lecture 8
... Calculate [OH‒] in a solution in which the concentration of protons is 0.0012 M at 25C. Is the solution acidic, basic or neutral? At 25C, Kw is always equal to 1 × 10-14 . Kw = [H3O+] [OH‒] 1 × 10-14 = (0.0012) [OH‒] [OH‒] = 1 × 10-14 / 0.0012 = 8.3 × 10-12 M The solution is acidic. ...
... Calculate [OH‒] in a solution in which the concentration of protons is 0.0012 M at 25C. Is the solution acidic, basic or neutral? At 25C, Kw is always equal to 1 × 10-14 . Kw = [H3O+] [OH‒] 1 × 10-14 = (0.0012) [OH‒] [OH‒] = 1 × 10-14 / 0.0012 = 8.3 × 10-12 M The solution is acidic. ...
Multiplets in Polymer Gels. Rare Earth Metal Ions Luminescence Study
... under the decay curves of Tb3+ 5D4 excited state with and without acceptor Eu3+ was shown to be equal to ca. 15%. In methanol solutions under the same conditions the decay curves for the mixture Eu(NO3)3/Tb(NO3)3 are fairly close to that for Tb(NO3)3 alone. This suggests that in the gel some of the ...
... under the decay curves of Tb3+ 5D4 excited state with and without acceptor Eu3+ was shown to be equal to ca. 15%. In methanol solutions under the same conditions the decay curves for the mixture Eu(NO3)3/Tb(NO3)3 are fairly close to that for Tb(NO3)3 alone. This suggests that in the gel some of the ...
Ionization of High-Density Deep Donor Defect States Explains the
... pyrite single crystals by CVT using iron (II) chloride as the transporting agent (100 mg /1 g of iron pyrite) in a sealed quartz ampule (14 mm inner diameter and 230 mm length), as shown in Figure 1a in the main text. The CVT crystal growth was conducted for a period of 3 weeks in a custom-built two ...
... pyrite single crystals by CVT using iron (II) chloride as the transporting agent (100 mg /1 g of iron pyrite) in a sealed quartz ampule (14 mm inner diameter and 230 mm length), as shown in Figure 1a in the main text. The CVT crystal growth was conducted for a period of 3 weeks in a custom-built two ...
Nanofluidic circuitry
Nanofluidic circuitry is a nanotechnology aiming for control of fluids in nanometer scale. Due to the effect of an electrical double layer within the fluid channel, the behavior of nanofluid is observed to be significantly different compared with its microfluidic counterparts. Its typical characteristic dimensions fall within the range of 1–100 nm. At least one dimension of the structure is in nanoscopic scale. Phenomena of fluids in nano-scale structure are discovered to be of different properties in electrochemistry and fluid dynamics.