Chapter 1 - TamAPChemistryHart
... air. The balloon thus weighs less than the air displaced by its volume. d) Because helium has a lower molar mass than the average air molecule, the helium atoms are in faster motion. This means that the temperature of the helium is higher than the air temperature. Hot gases tend to rise. ...
... air. The balloon thus weighs less than the air displaced by its volume. d) Because helium has a lower molar mass than the average air molecule, the helium atoms are in faster motion. This means that the temperature of the helium is higher than the air temperature. Hot gases tend to rise. ...
For the following mix equal volumes of one solution from Group I
... Group Activity: Due Wednesday, November 30, in class. For the following, mix equal volumes of one solution from Group I with one solution from Group II to achieve the indicated general pH. Then calculate the actual pH of the mixed solution (this requires setting up an I.C.E. table). a. b. c. ...
... Group Activity: Due Wednesday, November 30, in class. For the following, mix equal volumes of one solution from Group I with one solution from Group II to achieve the indicated general pH. Then calculate the actual pH of the mixed solution (this requires setting up an I.C.E. table). a. b. c. ...
Slide 1
... Distinguish between internal and external mixtures: internal mixture – two or more species in same particle external mixture – species found in separate particles in the same sample Relatively little known about the chemical form E.g., sulphates may occur as (NH4)2SO4 or (NH4)HSO4 Analyse particles ...
... Distinguish between internal and external mixtures: internal mixture – two or more species in same particle external mixture – species found in separate particles in the same sample Relatively little known about the chemical form E.g., sulphates may occur as (NH4)2SO4 or (NH4)HSO4 Analyse particles ...
Fig 2. - University of Warwick
... lives. Nature has carefully chosen our molecular building blocks so that the potentially devastating effects of ultraviolet radiation are by-passed. Some of the most important molecular building blocks, the DNA bases (adenine, thymine, guanine and cytosine), absorb ultraviolet radiation very readily ...
... lives. Nature has carefully chosen our molecular building blocks so that the potentially devastating effects of ultraviolet radiation are by-passed. Some of the most important molecular building blocks, the DNA bases (adenine, thymine, guanine and cytosine), absorb ultraviolet radiation very readily ...
C - Upton-by-Chester High School
... Thermal – using heat (1) To break down the compound (1) 2) What is produced when long alkanes are cracked and explain they are cracked. Short chain alkane (1) Short chain Alkenes (1) These molecules are in higher demand than long chain alkanes (1) 3) Why is the porous pot used in Cracking?Catalyst ...
... Thermal – using heat (1) To break down the compound (1) 2) What is produced when long alkanes are cracked and explain they are cracked. Short chain alkane (1) Short chain Alkenes (1) These molecules are in higher demand than long chain alkanes (1) 3) Why is the porous pot used in Cracking?Catalyst ...
Chapter 2: The Chemical Level Of Organization
... (CO2 is an exception because it contains carbon but is considered inorganic.) Water is the first inorganic compound listed in the table. As shown in the Functions column, water does more than just serve as a solvent for other molecules. In the form of blood, it delivers materials and heat; in the fo ...
... (CO2 is an exception because it contains carbon but is considered inorganic.) Water is the first inorganic compound listed in the table. As shown in the Functions column, water does more than just serve as a solvent for other molecules. In the form of blood, it delivers materials and heat; in the fo ...
Macromolecules webquest answer key
... publishing as Benjamin Cummings 3 5. Functional groups can modify the properties of organic molecules. In the table below, To read a set of chromosomes, scientists look for key features to identify their similarities and differences. Chem4TEENs.com! This tutorial introduces basics of biochemistry. O ...
... publishing as Benjamin Cummings 3 5. Functional groups can modify the properties of organic molecules. In the table below, To read a set of chromosomes, scientists look for key features to identify their similarities and differences. Chem4TEENs.com! This tutorial introduces basics of biochemistry. O ...
SC71 Chemistry
... Concept 2: Scientific Testing (Investigating and Modeling) Design and conduct controlled investigations. PO 1. Demonstrate safe and ethical procedures (e.g., use and care of technology, materials, organisms) and behavior in all science inquiry. PO 2. Identify the resources needed to conduct an inves ...
... Concept 2: Scientific Testing (Investigating and Modeling) Design and conduct controlled investigations. PO 1. Demonstrate safe and ethical procedures (e.g., use and care of technology, materials, organisms) and behavior in all science inquiry. PO 2. Identify the resources needed to conduct an inves ...
Introduction - Assets - Cambridge University Press
... from the melt zone of the Greenland ice cap and directly from Antarctic old blue ice. In the 50–100-µm size range, a constant high percentage of 80% of unmelted chondritic micrometeorites has been observed, indicating that many particles cross the terrestrial atmosphere without drastic thermal alter ...
... from the melt zone of the Greenland ice cap and directly from Antarctic old blue ice. In the 50–100-µm size range, a constant high percentage of 80% of unmelted chondritic micrometeorites has been observed, indicating that many particles cross the terrestrial atmosphere without drastic thermal alter ...
UNIT 2 – Chemical Quantities
... O2) would occur. CO2 is removed using the following chemical reaction: CO2(g) + 2LiOH(s) Li2CO3(g) + H2O(g) . If an astronaut produces 1.50x103 g of CO2 a day, what mass of LiOH would be needed per day? ...
... O2) would occur. CO2 is removed using the following chemical reaction: CO2(g) + 2LiOH(s) Li2CO3(g) + H2O(g) . If an astronaut produces 1.50x103 g of CO2 a day, what mass of LiOH would be needed per day? ...
Mass Spectrometry and Organic
... question. Molecular weight is not ambiguous. A compound has a unique MW. Our ability to analyze compounds on this basis, depends completely on being able to generate ions from the compound. Specifically molecular ions*, whose weight is equal to the MW of the compound, are critical. Once produced, ou ...
... question. Molecular weight is not ambiguous. A compound has a unique MW. Our ability to analyze compounds on this basis, depends completely on being able to generate ions from the compound. Specifically molecular ions*, whose weight is equal to the MW of the compound, are critical. Once produced, ou ...
mb_ch03
... Functional Groups • Functional groups are groups of atoms that influence the properties of molecules and the chemical reactions in which the molecules participate. ...
... Functional Groups • Functional groups are groups of atoms that influence the properties of molecules and the chemical reactions in which the molecules participate. ...
Ch 3 Notes
... Functional Groups • Functional groups are groups of atoms that influence the properties of molecules and the chemical reactions in which the molecules participate. ...
... Functional Groups • Functional groups are groups of atoms that influence the properties of molecules and the chemical reactions in which the molecules participate. ...
tutorial on carbohydrates
... 12. The polymer chains of glycosaminoglycans are widely spread apart and bind large amount of water. a. What 2 functional groups of the polymer make this binding of water possible? b. What type of binding is involved? 13. In glycoproteins, what are the 3 amino acids to which the carbohydrate groups ...
... 12. The polymer chains of glycosaminoglycans are widely spread apart and bind large amount of water. a. What 2 functional groups of the polymer make this binding of water possible? b. What type of binding is involved? 13. In glycoproteins, what are the 3 amino acids to which the carbohydrate groups ...
Polymers
... is expressed as the crosslink density As number of crosslinks increases, the glass transition temperature increases. ...
... is expressed as the crosslink density As number of crosslinks increases, the glass transition temperature increases. ...
Molecular Cell Biology
... Non-covalent bonds Several types: hydrogen bonds, ionic bonds, van der Waals interactions, hydrophobic bonds Non-covalent bonds require less energy to break than covalent bonds The energy required to break noncovalent bonds is only slightly greater than the average kinetic energy of molecules at ro ...
... Non-covalent bonds Several types: hydrogen bonds, ionic bonds, van der Waals interactions, hydrophobic bonds Non-covalent bonds require less energy to break than covalent bonds The energy required to break noncovalent bonds is only slightly greater than the average kinetic energy of molecules at ro ...
Dionex AminoPac Columns for the Analysis of Amino Acids
... • Amino acid content determination can be used to establish the primary structure of a protein or peptide. It is necessary to hydrolyze the protein of interest, and the choice of hydrolysis procedures is key to accurate analysis as some sensitive amino acids may be destroyed during the hydrolys ...
... • Amino acid content determination can be used to establish the primary structure of a protein or peptide. It is necessary to hydrolyze the protein of interest, and the choice of hydrolysis procedures is key to accurate analysis as some sensitive amino acids may be destroyed during the hydrolys ...
Cellular Metabolism and Nutrition notes
... – As enzyme concentration increases, enzyme activity will increase to a point. – As substrate concentration increases, enzyme activity will increase to a point. ...
... – As enzyme concentration increases, enzyme activity will increase to a point. – As substrate concentration increases, enzyme activity will increase to a point. ...
Molecular Geometry Why?
... Molecular Geometry How can molecular shapes be predicted using the VSEPR theory? ...
... Molecular Geometry How can molecular shapes be predicted using the VSEPR theory? ...
Fundamentals of Protein Chemistry and Mass Spectrometry
... AmBic pH 8.0 to give 10 ng/μL. Gel pieces should be just covered, but not in a large excess of volume (for a single 2D gel spot, use 25-30 μL of 10 ng/μL trypsin). Digest overnight for 16-18 hours at 37°C. Following digestion, centrifuge condensate to bottom of vial. Add extraction solution of 1% fo ...
... AmBic pH 8.0 to give 10 ng/μL. Gel pieces should be just covered, but not in a large excess of volume (for a single 2D gel spot, use 25-30 μL of 10 ng/μL trypsin). Digest overnight for 16-18 hours at 37°C. Following digestion, centrifuge condensate to bottom of vial. Add extraction solution of 1% fo ...
2A6
... Visible-light-induced molecular photodissociation of dimethyl disulfide ((CH3S)2) adsorbed on Ag(111) and Cu(111) surfaces was investigated by means of scanning tunneling microscopy (STM) combined with density functional theory (DFT) calculations. The visible-light-induced photodissociation on metal ...
... Visible-light-induced molecular photodissociation of dimethyl disulfide ((CH3S)2) adsorbed on Ag(111) and Cu(111) surfaces was investigated by means of scanning tunneling microscopy (STM) combined with density functional theory (DFT) calculations. The visible-light-induced photodissociation on metal ...
Chapter 5 of Zumdahl
... what volume of N2O collected over water at a total pressure of 94 kPa and 22ºC can be produced from 2.6 g of NH4NO3? ( the vapor pressure of water at 22ºC is 21 torr) ...
... what volume of N2O collected over water at a total pressure of 94 kPa and 22ºC can be produced from 2.6 g of NH4NO3? ( the vapor pressure of water at 22ºC is 21 torr) ...
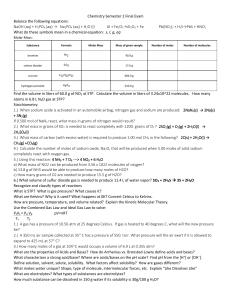
Chemistry Semester 2 Final Exam Chemistry Semester 2 Final Exam
... 1.) A gas has a pressure of 10.56 atm at 25 degrees Celcius. If gas is heated to 40 degrees C, what will the new pressure be? 10.0 atm 2.) A 350 mL air sample collected at 35 C has a pressure of 550. torr. What pressure will the air exert if it is allowed to expand to 425 mL at 57 C? 485 torr 3.) ...
... 1.) A gas has a pressure of 10.56 atm at 25 degrees Celcius. If gas is heated to 40 degrees C, what will the new pressure be? 10.0 atm 2.) A 350 mL air sample collected at 35 C has a pressure of 550. torr. What pressure will the air exert if it is allowed to expand to 425 mL at 57 C? 485 torr 3.) ...
Chemistry of Fats and Carbohydrates
... Chemistry of Fats and Carbohydrates All living things are composed of many different kinds of chemical molecules. Two very important chemical molecules are fats and proteins. Both make up parts of living cells. Fats are a part of all cellular membranes. They also may be stored within a cell as an en ...
... Chemistry of Fats and Carbohydrates All living things are composed of many different kinds of chemical molecules. Two very important chemical molecules are fats and proteins. Both make up parts of living cells. Fats are a part of all cellular membranes. They also may be stored within a cell as an en ...
Size-exclusion chromatography

Size-exclusion chromatography (SEC) is a chromatographic method in which molecules in solution are separated by their size, and in some cases molecular weight. It is usually applied to large molecules or macromolecular complexes such as proteins and industrial polymers. Typically, when an aqueous solution is used to transport the sample through the column, the technique is known as gel-filtration chromatography, versus the name gel permeation chromatography, which is used when an organic solvent is used as a mobile phase. SEC is a widely used polymer characterization method because of its ability to provide good molar mass distribution (Mw) results for polymers.