College Chemistry 1 Note Guide(free download)
... Universities and designates the course as being the college or university chemistry lecture course for majors in the field. The DVDs are copies of those distance education videos and are made available courtesy of Gulf Coast Community College. The workbook/note guide written by Dr. Etheridge is desi ...
... Universities and designates the course as being the college or university chemistry lecture course for majors in the field. The DVDs are copies of those distance education videos and are made available courtesy of Gulf Coast Community College. The workbook/note guide written by Dr. Etheridge is desi ...
Chemistry Summer Work (30 questions):
... Question #6. In every Periodic Table, even the most basic, one can find two numbers associated with each type of element. For example, for zinc (Zn) those numbers are 30 and 65.409. Explain what these numbers represent. Give units if the numbers have units. Question #7. 55Fe is a radioactive isotop ...
... Question #6. In every Periodic Table, even the most basic, one can find two numbers associated with each type of element. For example, for zinc (Zn) those numbers are 30 and 65.409. Explain what these numbers represent. Give units if the numbers have units. Question #7. 55Fe is a radioactive isotop ...
Human Biology The Chemistry of Living Things 2.1 Multiple Choice
... 35) Pancreatic cells make insulin, which is a type of protein. These cells use ________ in order to synthesize insulin by the process of ________. A) oligosaccharides, hydrolysis B) nucleotides, condensation C) amino acids, dehydration synthesis D) fatty acids and glycerol, hydrolysis E) monosacchar ...
... 35) Pancreatic cells make insulin, which is a type of protein. These cells use ________ in order to synthesize insulin by the process of ________. A) oligosaccharides, hydrolysis B) nucleotides, condensation C) amino acids, dehydration synthesis D) fatty acids and glycerol, hydrolysis E) monosacchar ...
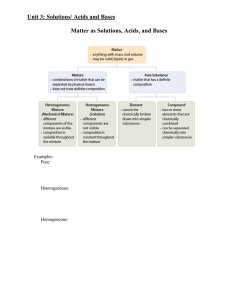
Outline for Unit 1 Solutions, Acid/Base, and Gases
... 4. Particle size – smaller particles dissolve faster since there is more surface area available to solvent 5. Pressure (partial pressure) – only affects gas in liquids – solubility of gas increases as partial pressure of the gas above the solution is increased. ...
... 4. Particle size – smaller particles dissolve faster since there is more surface area available to solvent 5. Pressure (partial pressure) – only affects gas in liquids – solubility of gas increases as partial pressure of the gas above the solution is increased. ...
acetyl CoA
... • The respiratory chain is a sequence of redox reactions, during which proteins in different complexes accept electrons and donate them immediately to the next complex. • Oxygen is the final electron acceptor. ...
... • The respiratory chain is a sequence of redox reactions, during which proteins in different complexes accept electrons and donate them immediately to the next complex. • Oxygen is the final electron acceptor. ...
Physical Chemistry II
... test your conceptual understanding of the subject matter Rapid quizzes are provided to check your understanding Practical experiments will be given to evaluate your understanding of theory-practice relations Simulated experiments/exercise will give to test your understanding of certain concepts Lear ...
... test your conceptual understanding of the subject matter Rapid quizzes are provided to check your understanding Practical experiments will be given to evaluate your understanding of theory-practice relations Simulated experiments/exercise will give to test your understanding of certain concepts Lear ...
Default Normal Template
... Dalton’s law of partial pressure: The total pressure exerted by a mixture of gases, that do not interact, equals sum of partial pressures of all gases, if each gas occupies the container on its own. P total = P1 + P2 + P3 + ………… ...
... Dalton’s law of partial pressure: The total pressure exerted by a mixture of gases, that do not interact, equals sum of partial pressures of all gases, if each gas occupies the container on its own. P total = P1 + P2 + P3 + ………… ...
2010 Exam
... The items below are your responsibility. Please ensure that they are completed. Write your name and teacher’s name on the top of this page. Write your name, teacher’s name, course name and number on the Part I answer sheet. Check the exam to see that there are no missing pages. ...
... The items below are your responsibility. Please ensure that they are completed. Write your name and teacher’s name on the top of this page. Write your name, teacher’s name, course name and number on the Part I answer sheet. Check the exam to see that there are no missing pages. ...
Porous silicon-based nanostructured microparticles as degradable
... round-bottom flask containing 50 mL of anhydrous THF, and the mixture was heated at reflux for 30 minutes under N2. Heating was stopped and a solution of 9-undecenamide (5.0 g, 27.3 mmol) in 100 mL of anhydrous THF was introduced with stirring. The solution was refluxed under N2 for another 24 hours ...
... round-bottom flask containing 50 mL of anhydrous THF, and the mixture was heated at reflux for 30 minutes under N2. Heating was stopped and a solution of 9-undecenamide (5.0 g, 27.3 mmol) in 100 mL of anhydrous THF was introduced with stirring. The solution was refluxed under N2 for another 24 hours ...
Purification and characterization of the 1-3
... Molecular weight of the native enzyme was determined by two methods: 1. Gel filtration on a FPLC column of superose 12 HR 10/30 equilibrated with 50 mM KPB (pH 7.4) containing 100 mM KCl. Column was calibrated with known molecular weight standards (Da) (Sigma, St. Louis, MO, USA): blue dextran (2 00 ...
... Molecular weight of the native enzyme was determined by two methods: 1. Gel filtration on a FPLC column of superose 12 HR 10/30 equilibrated with 50 mM KPB (pH 7.4) containing 100 mM KCl. Column was calibrated with known molecular weight standards (Da) (Sigma, St. Louis, MO, USA): blue dextran (2 00 ...
Measurement and data processing and analysis
... significant figures of the measurement. There are two significant figures, for example, in 62 cm3 and five in 100.00 g. The zeros are significant here as they signify that the uncertainty range is (±) 0.01 g. The number of significant figures may not always be clear. If a time measurement is 1000 s, ...
... significant figures of the measurement. There are two significant figures, for example, in 62 cm3 and five in 100.00 g. The zeros are significant here as they signify that the uncertainty range is (±) 0.01 g. The number of significant figures may not always be clear. If a time measurement is 1000 s, ...
Calculations - The Student Room
... From the relative molecular mass (Mr) work out how many times the mass of the empirical formula fits into the Mr. ...
... From the relative molecular mass (Mr) work out how many times the mass of the empirical formula fits into the Mr. ...
The CoFactor database: organic cofactors in enzyme catalysis
... Enzymes are proteins that catalyze the repertoire of chemical reactions found in nature, and as such are vitally important molecules. They are generally composed of the 20 common amino acid residues, but many also require small molecules in addition for the catalysis to occur. In some cases, these m ...
... Enzymes are proteins that catalyze the repertoire of chemical reactions found in nature, and as such are vitally important molecules. They are generally composed of the 20 common amino acid residues, but many also require small molecules in addition for the catalysis to occur. In some cases, these m ...
Chapter #3
... 1.027 g CO2 and 0.4194 g H2O. From this data calculate the molecular formula. Plan: We find the masses of Hydrogen and Carbon using the mass fractions of H in H2O, and C in CO2. The mass of Carbon and Hydrogen are subtracted from the sample mass to get the mass of Oxygen. We then calculate moles, an ...
... 1.027 g CO2 and 0.4194 g H2O. From this data calculate the molecular formula. Plan: We find the masses of Hydrogen and Carbon using the mass fractions of H in H2O, and C in CO2. The mass of Carbon and Hydrogen are subtracted from the sample mass to get the mass of Oxygen. We then calculate moles, an ...
Lab: Colony PCR amplification of the 16S ribosomal RNA gene I
... 3. Seal the ends of the gel form to prepare it for pouring. In some cases, the gel forms are sealed by their gasketed ends in the gel bed; in others, they have an external device for sealing the ends of the form. It is unique for each gel setup, so if you are unsure ask for assistance. Once the gel ...
... 3. Seal the ends of the gel form to prepare it for pouring. In some cases, the gel forms are sealed by their gasketed ends in the gel bed; in others, they have an external device for sealing the ends of the form. It is unique for each gel setup, so if you are unsure ask for assistance. Once the gel ...
Gr. 11 Chemistry Student Workbook (Spring 2016)
... An active science program presents some hazards to both staff and students. All attempts will be made however, to identify hazards and manage risks so that they become minimal. Before each activity, instructions will be given to reduce any risks. Teachers will assess the readiness level of students ...
... An active science program presents some hazards to both staff and students. All attempts will be made however, to identify hazards and manage risks so that they become minimal. Before each activity, instructions will be given to reduce any risks. Teachers will assess the readiness level of students ...
Physical Chemistry 2.pdf
... The module, Physical Chemistry 2, focuses on five (5) areas of physical chemistry important to many aspects of our lives: solutions, colloids, phase equilibrium, electrochemistry and nuclear chemistry. Solutions are often necessary to facilitate many chemical reactions in life processes or industry ...
... The module, Physical Chemistry 2, focuses on five (5) areas of physical chemistry important to many aspects of our lives: solutions, colloids, phase equilibrium, electrochemistry and nuclear chemistry. Solutions are often necessary to facilitate many chemical reactions in life processes or industry ...
1 3. Molecular mass transport 3.1 Introduction to mass transfer 3.2
... rate of processes accruing in liquids (such as reaction between two components in liquids). In chemistry, diffusivity limits the rate of acid-base reactions; in the chemical industry, diffusion is responsible for the rates of liquid-liquid extraction. Diffusion in liquids is important because it is ...
... rate of processes accruing in liquids (such as reaction between two components in liquids). In chemistry, diffusivity limits the rate of acid-base reactions; in the chemical industry, diffusion is responsible for the rates of liquid-liquid extraction. Diffusion in liquids is important because it is ...
1. Sucrose is a disaccharide. The diagram shows the structure of a
... the term used to refer to the sequence of amino acids which makes up a particular protein. These amino acids are linked by peptide bonds. The side-chains or R-groups of different amino acids may form chemical bonds with each other. It is these bonds which allow the formation of protein molecules wit ...
... the term used to refer to the sequence of amino acids which makes up a particular protein. These amino acids are linked by peptide bonds. The side-chains or R-groups of different amino acids may form chemical bonds with each other. It is these bonds which allow the formation of protein molecules wit ...
CfE HIGHER CHEMISTRY Chemistry in Society
... When reactants and products are gaseous it is not always possible to measure masses of gases; they are too difficult to weigh. It is much easier to deal with volumes of gases. We still have to relate gas volumes to numbers of moles. So what is the volume of a mole of gas and does it depend on which ...
... When reactants and products are gaseous it is not always possible to measure masses of gases; they are too difficult to weigh. It is much easier to deal with volumes of gases. We still have to relate gas volumes to numbers of moles. So what is the volume of a mole of gas and does it depend on which ...
Unit 1 Ch. 2,3,4 notes NEW
... Molar Mass Calculations eg. A sample of Sn contains 4.69 x 1028 atoms. Calculate its mass. ...
... Molar Mass Calculations eg. A sample of Sn contains 4.69 x 1028 atoms. Calculate its mass. ...
Chemistry II Honors – Unit 3 Study Guide
... Iron is biologically important in the transport of oxygen by red blood cells from the lungs to the various organs of the body. In the blood of an adult human, there are approximately 2.65e13 red blood cells with a total of 2.90 g of iron. On the average, how many iron atoms are present in each red b ...
... Iron is biologically important in the transport of oxygen by red blood cells from the lungs to the various organs of the body. In the blood of an adult human, there are approximately 2.65e13 red blood cells with a total of 2.90 g of iron. On the average, how many iron atoms are present in each red b ...
CHEMISTRY 2202 UNIT 1 STOICHIOMETRY
... eg 4: A chemist isolates 2.99 g of a gas. The sample occupies 800.0 mL at STP. Is the gas most likely to be O2(g), Kr(g), Ne(g), or F2(g)? (Hint: Determine the molar mass of the gas.) ...
... eg 4: A chemist isolates 2.99 g of a gas. The sample occupies 800.0 mL at STP. Is the gas most likely to be O2(g), Kr(g), Ne(g), or F2(g)? (Hint: Determine the molar mass of the gas.) ...
Chapter 3
... atoms are very very small particles and we can not count it or weight it easily that because it contains huge number of atoms. For example the smallest thing we can see by our nicked eyes contains about 1016 atom, it is huge number is not?!!!! It is clear that we can not weight a single atom, but as ...
... atoms are very very small particles and we can not count it or weight it easily that because it contains huge number of atoms. For example the smallest thing we can see by our nicked eyes contains about 1016 atom, it is huge number is not?!!!! It is clear that we can not weight a single atom, but as ...
AP Chemistry - Oak Park Unified School District
... All measured quantities are inexact to some extent. The (7) of a measurement indicates how closely different measurements of a quantity agree with one another. The (8) of a measurement indicates how well a measurement agrees with the accepted value. Significant figures indicate the level of (9) in a ...
... All measured quantities are inexact to some extent. The (7) of a measurement indicates how closely different measurements of a quantity agree with one another. The (8) of a measurement indicates how well a measurement agrees with the accepted value. Significant figures indicate the level of (9) in a ...
Size-exclusion chromatography

Size-exclusion chromatography (SEC) is a chromatographic method in which molecules in solution are separated by their size, and in some cases molecular weight. It is usually applied to large molecules or macromolecular complexes such as proteins and industrial polymers. Typically, when an aqueous solution is used to transport the sample through the column, the technique is known as gel-filtration chromatography, versus the name gel permeation chromatography, which is used when an organic solvent is used as a mobile phase. SEC is a widely used polymer characterization method because of its ability to provide good molar mass distribution (Mw) results for polymers.