chapter2 2012 (no naming) 2014
... their bonds but atoms are not created or destroyed 4. Compounds are formed when two or more atoms of different element combine ...
... their bonds but atoms are not created or destroyed 4. Compounds are formed when two or more atoms of different element combine ...
chapter2 2012 (no naming)
... their bonds but atoms are not created or destroyed 4. Compounds are formed when two or more atoms of different element combine ...
... their bonds but atoms are not created or destroyed 4. Compounds are formed when two or more atoms of different element combine ...
LIST OF TOPICS COVERED DURING THIS COURSE
... The following should serve as a checklist for your notebook. The topics below include all topics that have been covered this semester and are testable on your final exam. These topics should be studied from a variety of source including inclass notes, homework questions, lab questions, assignments, ...
... The following should serve as a checklist for your notebook. The topics below include all topics that have been covered this semester and are testable on your final exam. These topics should be studied from a variety of source including inclass notes, homework questions, lab questions, assignments, ...
Note 1.1 Chemistry of Life
... lose or gain electrons to complete their valence shells. When metals and non metals combine, they form ionic compounds. When non metals combine, they share electrons to complete their valence shells, and form covalent bonds. They form hybridized electron orbitals. Biological molecules are created wh ...
... lose or gain electrons to complete their valence shells. When metals and non metals combine, they form ionic compounds. When non metals combine, they share electrons to complete their valence shells, and form covalent bonds. They form hybridized electron orbitals. Biological molecules are created wh ...
Stoich Powerpoint Review
... • Sometimes they will give you molar mass of a compound and its empirical formula. • You must find the “empirical formula mass” and divide the molar mass by the efm. ...
... • Sometimes they will give you molar mass of a compound and its empirical formula. • You must find the “empirical formula mass” and divide the molar mass by the efm. ...
Review for Midyear - 1 KEY - Ms. Robbins` PNHS Science Classes
... when pure, are crystalline salts at room temperature (common examples include NaCl, KI, Fe 2O3); and substances that are liquids and gases at room temperature are usually made of molecules that have covalent bonds (common examples include CO2, N2, CH4, H2O, C8H18) HS-PS1-7. Use mathematical represen ...
... when pure, are crystalline salts at room temperature (common examples include NaCl, KI, Fe 2O3); and substances that are liquids and gases at room temperature are usually made of molecules that have covalent bonds (common examples include CO2, N2, CH4, H2O, C8H18) HS-PS1-7. Use mathematical represen ...
10th Grade Chemistry X (TJ) GRADE(S)/LEVELS SUBJECT Power
... When elements are listed in order according to the number of protons, repeating patterns of physical and chemical properties identify families of elements with similar properties. This Periodic Table is a consequence of the repeating pattern of outermost electrons. LT 1 Predict the properties of ele ...
... When elements are listed in order according to the number of protons, repeating patterns of physical and chemical properties identify families of elements with similar properties. This Periodic Table is a consequence of the repeating pattern of outermost electrons. LT 1 Predict the properties of ele ...
Chap 2.1 Notes - Nature of Matter
... H and O are covalently bonded. In water – oxygen is “selfish” and does not share its electrons equally with hydrogen. Because of this - water is a polar compound - the molecule has an uneven electrical charge. Ex) Water acts like magnet with a “+” and “-“ pole. ...
... H and O are covalently bonded. In water – oxygen is “selfish” and does not share its electrons equally with hydrogen. Because of this - water is a polar compound - the molecule has an uneven electrical charge. Ex) Water acts like magnet with a “+” and “-“ pole. ...
Document
... A solid has a mass of 20g. When it is mixed with a solution a chemical reaction occurs in which a gas is produced. If the final total mass of the products is 55g, what was the mass of the solution? 20 g + solution = 55g 55g - 20g = mass of solution 35g = mass of solution ...
... A solid has a mass of 20g. When it is mixed with a solution a chemical reaction occurs in which a gas is produced. If the final total mass of the products is 55g, what was the mass of the solution? 20 g + solution = 55g 55g - 20g = mass of solution 35g = mass of solution ...
Statistical Computing and Simulation
... could count “nlminb” as one of the method (for replacing Newton’s method). Also, similar to what we saw in the class, discuss what is the influence of starting points to the number of iterations. Experiment with as many variance reduction techniques as you can think of to apply the problem of evalua ...
... could count “nlminb” as one of the method (for replacing Newton’s method). Also, similar to what we saw in the class, discuss what is the influence of starting points to the number of iterations. Experiment with as many variance reduction techniques as you can think of to apply the problem of evalua ...
4. - period2chem
... STRATEGY: Start by reading through your notes to refresh your memory on these topics. Then, use this review sheet as a starting point to identify the areas on which you need to spend more study time. For those areas, go back to homework assignments, quizzes, and reviews to practice more problems. I ...
... STRATEGY: Start by reading through your notes to refresh your memory on these topics. Then, use this review sheet as a starting point to identify the areas on which you need to spend more study time. For those areas, go back to homework assignments, quizzes, and reviews to practice more problems. I ...
Exam 3 Review
... The symbol for the magnetic quantum number is m which defines the orbital. m = - , (- + 1), (- +2), .....0, ......., ( -2), ( -1), The last quantum number is the spin quantum number which has the symbol m s which characterizes the single electron. The spin quantum number only has two pos ...
... The symbol for the magnetic quantum number is m which defines the orbital. m = - , (- + 1), (- +2), .....0, ......., ( -2), ( -1), The last quantum number is the spin quantum number which has the symbol m s which characterizes the single electron. The spin quantum number only has two pos ...
chemistry - cloudfront.net
... Which of the following observations is evidence used to imply that a chemical reaction has occurred? a – f a. gas evolution b. a precipitate forms (solid formed from aqueous solutions) c. the color changes d. the temperature changes e. the odor changes f. a sound is produced (i.e. fireworks) g. ch ...
... Which of the following observations is evidence used to imply that a chemical reaction has occurred? a – f a. gas evolution b. a precipitate forms (solid formed from aqueous solutions) c. the color changes d. the temperature changes e. the odor changes f. a sound is produced (i.e. fireworks) g. ch ...
2A Final Exam Review Worksheet
... o Temperature is proportional to kinetic energy. Two molecules at the same temperature will have the same average kinetic energy. o If two molecules are under the same conditions, the heavier molecule will travel slower ...
... o Temperature is proportional to kinetic energy. Two molecules at the same temperature will have the same average kinetic energy. o If two molecules are under the same conditions, the heavier molecule will travel slower ...
Chemical reaction
... • Solute – the substance dissolved in the solution (Sugar) • Solvent – the substance in which the solute is dissolved (Water)[aqueous] ...
... • Solute – the substance dissolved in the solution (Sugar) • Solvent – the substance in which the solute is dissolved (Water)[aqueous] ...
Document
... 22. Numbers that precede symbols and formulas in a chemical equation are ______________. 23. A chemical reaction in which two or more substances combine to form another substance is called a ___________________. 24. According to the law of conservation of mass, if two atoms of hydrogen are on the re ...
... 22. Numbers that precede symbols and formulas in a chemical equation are ______________. 23. A chemical reaction in which two or more substances combine to form another substance is called a ___________________. 24. According to the law of conservation of mass, if two atoms of hydrogen are on the re ...
On the Development of Atomic Theory
... far ahead of his time. One cannot but add here that the first really scientific progress beyond some of his ideas was made almost 2,000 years later. Indeed, for a continuous space of time very little more was heard about atoms. It was not until the year 1808 that John Dalton, a well-known English ch ...
... far ahead of his time. One cannot but add here that the first really scientific progress beyond some of his ideas was made almost 2,000 years later. Indeed, for a continuous space of time very little more was heard about atoms. It was not until the year 1808 that John Dalton, a well-known English ch ...
File
... valence shell) usually determine how an atom will react Atoms are stable when their outer energy level is full Atoms can gain or lose electrons to become stable ...
... valence shell) usually determine how an atom will react Atoms are stable when their outer energy level is full Atoms can gain or lose electrons to become stable ...
Chapter 5
... General properties of ionic bonding; formation of binary ionic compounds Ionic bonding and dot structures Lattice energy; definition; lattice energy and Coulomb’s law Trends in lattice energy (ion size, ion charge); explanation for trends Failure of ionic bonding of nonmetals with nonmetals Covalent ...
... General properties of ionic bonding; formation of binary ionic compounds Ionic bonding and dot structures Lattice energy; definition; lattice energy and Coulomb’s law Trends in lattice energy (ion size, ion charge); explanation for trends Failure of ionic bonding of nonmetals with nonmetals Covalent ...
Review for second exam:
... General properties of ionic bonding; formation of binary ionic compounds Ionic bonding and dot structures Lattice energy; definition; lattice energy and Coulomb’s law Trends in lattice energy (ion size, ion charge); explanation for trends Failure of ionic bonding of nonmetals with nonmetals Covalent ...
... General properties of ionic bonding; formation of binary ionic compounds Ionic bonding and dot structures Lattice energy; definition; lattice energy and Coulomb’s law Trends in lattice energy (ion size, ion charge); explanation for trends Failure of ionic bonding of nonmetals with nonmetals Covalent ...
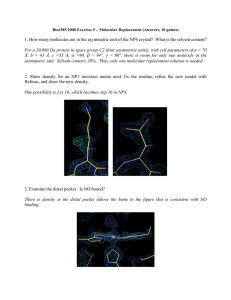
1. How many molecules are in the asymmetric unit of the NP4 crystal
... heme. The best justification would be to rebuild the loop into the extra density and refine the structure to see if the fit is reasonable (not required). ...
... heme. The best justification would be to rebuild the loop into the extra density and refine the structure to see if the fit is reasonable (not required). ...
Chemistry Study Guide
... Table of elements arranged by their atomic number – the number of protons. An elements position on the table will show many of its general properties Periods- The table is arranged in horizontal rows called periods. The period tells you how many electron energy levels the atom has. Groups- Verti ...
... Table of elements arranged by their atomic number – the number of protons. An elements position on the table will show many of its general properties Periods- The table is arranged in horizontal rows called periods. The period tells you how many electron energy levels the atom has. Groups- Verti ...