Chemistry - cloudfront.net
... 84. Understand that matter can be converted to Energy by either fission or fusion processes and this energy is given by the equation E=mc2 [note: m must be in kilograms; E will come out in Joules] 85. Given a nuclear reaction, be able to determine the missing particle (alpha, beta or positron) that ...
... 84. Understand that matter can be converted to Energy by either fission or fusion processes and this energy is given by the equation E=mc2 [note: m must be in kilograms; E will come out in Joules] 85. Given a nuclear reaction, be able to determine the missing particle (alpha, beta or positron) that ...
Building the sense of math in physics activities
... divided by the viscous force, Ffluid→ filter = 6πμ Rv where μ is the viscosity of the fluid, R is the radius of the object and v is its velocity through the fluid. (This is actually correct up to a dimensionless factor. For this problem take Re to be the ratio of these two forces.) B.1 Write an equa ...
... divided by the viscous force, Ffluid→ filter = 6πμ Rv where μ is the viscosity of the fluid, R is the radius of the object and v is its velocity through the fluid. (This is actually correct up to a dimensionless factor. For this problem take Re to be the ratio of these two forces.) B.1 Write an equa ...
General Chemistry (First Semester)
... ◊ Constructing a histogram for numerical data ♦ Electrostatics (5 topics) ◊ Understanding that opposite charges attract and like charges repel ◊ Understanding how electrostatic force scales with charge and separation ◊ Understanding how electrostatic forces cancel ◊ Understanding how electrostatic e ...
... ◊ Constructing a histogram for numerical data ♦ Electrostatics (5 topics) ◊ Understanding that opposite charges attract and like charges repel ◊ Understanding how electrostatic force scales with charge and separation ◊ Understanding how electrostatic forces cancel ◊ Understanding how electrostatic e ...
Chemistry 101: The Complete Notes
... Result: Successful chemistry students typically spend most of their independent study time working assigned problems, not just reading about them. To learn chemistry you must do chemistry is a truism worth remembering. An analogy would be this: you read all the books out there on the subject of golf ...
... Result: Successful chemistry students typically spend most of their independent study time working assigned problems, not just reading about them. To learn chemistry you must do chemistry is a truism worth remembering. An analogy would be this: you read all the books out there on the subject of golf ...
Slides
... EC 3 : hydrolases (enzymes that use water to break up some other molecules ) EC 3.4 : hydrolases that act on peptide bonds EC 3.4.11 : hydrolases that cleave off the aminoterminal amino acid from polypeptide EC 3.4.11.4 : hydrolases that cleave off the aminoReference: terminal end from a tripeptide ...
... EC 3 : hydrolases (enzymes that use water to break up some other molecules ) EC 3.4 : hydrolases that act on peptide bonds EC 3.4.11 : hydrolases that cleave off the aminoterminal amino acid from polypeptide EC 3.4.11.4 : hydrolases that cleave off the aminoReference: terminal end from a tripeptide ...
The Mole: A Measurement of Matter
... from Chapter 4 that this is because atomic masses are weighted average masses of the isotopes of each element. Quantities measured in grams are convenient for working in the laboratory, so chemists have converted the relative scale of masses of the elements in amu to a relative scale of masses in gr ...
... from Chapter 4 that this is because atomic masses are weighted average masses of the isotopes of each element. Quantities measured in grams are convenient for working in the laboratory, so chemists have converted the relative scale of masses of the elements in amu to a relative scale of masses in gr ...
Fall 2013 Final practice questions w/o solution
... promoted from n = 2 to n = 3 is less than that required to promote an electron from n = 3 to n = 4. E) Once an electron is promoted from one energy level to another, it is "stuck" there and cannot return to the ground state. ...
... promoted from n = 2 to n = 3 is less than that required to promote an electron from n = 3 to n = 4. E) Once an electron is promoted from one energy level to another, it is "stuck" there and cannot return to the ground state. ...
Chemistry Log Books - Social Circle City Schools
... 1. Students will receive an AKS review sheet for the upcoming unit (usually after the last unit test). This sheet should then be glued/taped to fit the page in the composition log book. Students will read the AKS listed at the top of the page to see what they will be learning in the upcoming unit. 2 ...
... 1. Students will receive an AKS review sheet for the upcoming unit (usually after the last unit test). This sheet should then be glued/taped to fit the page in the composition log book. Students will read the AKS listed at the top of the page to see what they will be learning in the upcoming unit. 2 ...
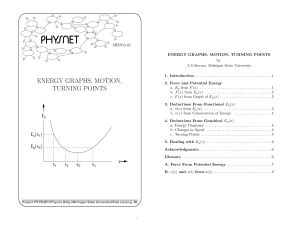
energy graphs, motion, turning points
... Kinetic energy is the difference between the total and potential energies. right or to the left. However, the particle’s change in kinetic energy, and hence its change in speed, does depend on its direction of motion. For example, if the particle in Fig. 2 is at xA and is moving to the right, its ki ...
... Kinetic energy is the difference between the total and potential energies. right or to the left. However, the particle’s change in kinetic energy, and hence its change in speed, does depend on its direction of motion. For example, if the particle in Fig. 2 is at xA and is moving to the right, its ki ...
Science Focus 9 Matter and Chemical Change Class Notes Topic 1
... be broken down any further, to determine if a substance was a pure substance or a mixture. Antoine Lavoisier defined elements as pure substances that could not be decomposed into simpler substances by means of a chemical change. In this way he identified 23 pure substances as elements. Lavoisier was ...
... be broken down any further, to determine if a substance was a pure substance or a mixture. Antoine Lavoisier defined elements as pure substances that could not be decomposed into simpler substances by means of a chemical change. In this way he identified 23 pure substances as elements. Lavoisier was ...
Chemistry - Plymouth Public Schools
... Central Concept: Physical and chemical properties reflect the nature of the interactions between molecules or atoms, and can be used to classify and describe matter. MA CHM 1.1 Identify and explain physical properties (e.g., density, melting point, boiling point, conductivity, malleability) and chem ...
... Central Concept: Physical and chemical properties reflect the nature of the interactions between molecules or atoms, and can be used to classify and describe matter. MA CHM 1.1 Identify and explain physical properties (e.g., density, melting point, boiling point, conductivity, malleability) and chem ...
Radiocommunication Study Groups
... continuous atomic time scale based on TAI with simultaneous broadcasting these two reference timescales (current UTC and continuous atomic time scale) on equal basis. However, practical implementation of Methods A and B seems difficult due to: - in case of Method A the problems related to disseminat ...
... continuous atomic time scale based on TAI with simultaneous broadcasting these two reference timescales (current UTC and continuous atomic time scale) on equal basis. However, practical implementation of Methods A and B seems difficult due to: - in case of Method A the problems related to disseminat ...
1 Exam Study Guide Chapter 11 Net Ionic Equations Precipitate
... -Closed container: no particles can escape so the particles at the surface vaporize and become vapor particles. As the number of vapor particles increase, some condense and become a liquid. Equilibrium is when the ratio of vaporization equals the rate of condensation, so that vapor pressure remains ...
... -Closed container: no particles can escape so the particles at the surface vaporize and become vapor particles. As the number of vapor particles increase, some condense and become a liquid. Equilibrium is when the ratio of vaporization equals the rate of condensation, so that vapor pressure remains ...
Document
... There are three significant reasons to study chemistry. First chemistry has important practical application in the society. The development of life saving drugs in one and a complete list would touch upon most areas of modern technology. Second chemistry is an intellectual enterprise, a way of expl ...
... There are three significant reasons to study chemistry. First chemistry has important practical application in the society. The development of life saving drugs in one and a complete list would touch upon most areas of modern technology. Second chemistry is an intellectual enterprise, a way of expl ...
AP Chem Stoichiometry Notes Table of Contents Atomic Masses
... o Sample Exercise 3.14 – Balancing a Chemical Equation I Chromium compounds exhibit a variety of bright colors. When solid ammonium dichromate, (NH4)2Cr2O7, a vivid orange compound, is ignited, a spectacular reaction occurs. Although the reaction is actually somewhat more complex, let’s assume here ...
... o Sample Exercise 3.14 – Balancing a Chemical Equation I Chromium compounds exhibit a variety of bright colors. When solid ammonium dichromate, (NH4)2Cr2O7, a vivid orange compound, is ignited, a spectacular reaction occurs. Although the reaction is actually somewhat more complex, let’s assume here ...
B) Examples of Avagadro`s Number
... XIII. The Limiting Reactant (Reagent) A) Most chemical reactions will continue until one of the reactants is completely used up--then, no more product(s) can be formed B) The reactant that is used up first, and therefore controls how much product is formed, is called the limiting reactant (or limit ...
... XIII. The Limiting Reactant (Reagent) A) Most chemical reactions will continue until one of the reactants is completely used up--then, no more product(s) can be formed B) The reactant that is used up first, and therefore controls how much product is formed, is called the limiting reactant (or limit ...
Balancing Chemical Equations Guided Inquiry (CC)
... destroyed. In fact, matter can never be created or destroyed. This is one of the basic principles in chemistry known as the Law of Conservation of Mass. Matter cannot be created or destroyed, but we can change its form. In order for the equation to make sense, we need to balance the equation. This c ...
... destroyed. In fact, matter can never be created or destroyed. This is one of the basic principles in chemistry known as the Law of Conservation of Mass. Matter cannot be created or destroyed, but we can change its form. In order for the equation to make sense, we need to balance the equation. This c ...
Chemistry Stoichiometry Standard Set 3 Review
... assigned to elements, the mass of 12 grams of carbon-12 was selected as a standard reference to which the masses of all other elements are compared. The number of atoms in 12 grams of carbon-12 is defined as one mole, or conversely, if one mole of 12C atoms were weighed, it would weigh exactly 12 gr ...
... assigned to elements, the mass of 12 grams of carbon-12 was selected as a standard reference to which the masses of all other elements are compared. The number of atoms in 12 grams of carbon-12 is defined as one mole, or conversely, if one mole of 12C atoms were weighed, it would weigh exactly 12 gr ...
Document
... during a process • if the final condition has a larger amount of internal energy than the initial condition, the change in the internal energy will be + • if the final condition has a smaller amount of internal energy than the initial condition, the change in the internal energy will be ─ ...
... during a process • if the final condition has a larger amount of internal energy than the initial condition, the change in the internal energy will be + • if the final condition has a smaller amount of internal energy than the initial condition, the change in the internal energy will be ─ ...
Data: I am writing out the question and underlining it.
... deteriorated, and what percent of our atmosphere is made up harmful pollutants? Well when fossil fuels are burned, or maybe even things like wood or who knows, scientists most likely calculate the molecules given off so they can come up with these statistics. Well maybe they deal with moles or liter ...
... deteriorated, and what percent of our atmosphere is made up harmful pollutants? Well when fossil fuels are burned, or maybe even things like wood or who knows, scientists most likely calculate the molecules given off so they can come up with these statistics. Well maybe they deal with moles or liter ...
Numerical Resolution Of The Schrödinger Equation
... In this equation, V (r, t) is the (local) potential, and − 2m these two terms is of course the Hamiltonian of the particle. In the following, we choose our system of units such that the mass of the particle m becomes the unit of mass, and ~ becomes the unit of angular momentum. Furthermore, we alway ...
... In this equation, V (r, t) is the (local) potential, and − 2m these two terms is of course the Hamiltonian of the particle. In the following, we choose our system of units such that the mass of the particle m becomes the unit of mass, and ~ becomes the unit of angular momentum. Furthermore, we alway ...
Spring 2008
... According to the Heisenberg uncertainty principle, if the uncertainty in the speed of an electron is 3.5 × 103 m/s, the uncertainty in its position (in m) is at least A. ...
... According to the Heisenberg uncertainty principle, if the uncertainty in the speed of an electron is 3.5 × 103 m/s, the uncertainty in its position (in m) is at least A. ...
Chapter 3 Chemical Reactions and Reaction Stoichiometry
... from the mass of CO2 produced (12.01 g C per 44.01 g CO2). Ø All H from the sample is converted to H2O; so the mass of H in the sample is found from the mass of H2O produced (2.02 g H per 18.02 g H2O). Ø If needed, the mass of a 3rd element (such as O or N) in the sample is found by subtracting ...
... from the mass of CO2 produced (12.01 g C per 44.01 g CO2). Ø All H from the sample is converted to H2O; so the mass of H in the sample is found from the mass of H2O produced (2.02 g H per 18.02 g H2O). Ø If needed, the mass of a 3rd element (such as O or N) in the sample is found by subtracting ...
Chapter 3 Chemical Compounds
... Binary molecular compounds are compounds made of two types of atoms (binary) that form molecules. In binary molecular compounds, the two types of atoms are nonmetals (or sometimes metalloids). The general rule for naming binary compounds is to list the name of the first element and then list the nam ...
... Binary molecular compounds are compounds made of two types of atoms (binary) that form molecules. In binary molecular compounds, the two types of atoms are nonmetals (or sometimes metalloids). The general rule for naming binary compounds is to list the name of the first element and then list the nam ...