Direct Determination of Metal Cyanides by Ion
... range. Plot the peak area for each metal cyanide complex versus the concentration injected and use a linear regression to fit the data. Table 4 summarizes the calibration data for a typical calibration curve obtained by injecting calibration standards covering the ranges shown. The calibration curve ...
... range. Plot the peak area for each metal cyanide complex versus the concentration injected and use a linear regression to fit the data. Table 4 summarizes the calibration data for a typical calibration curve obtained by injecting calibration standards covering the ranges shown. The calibration curve ...
Concrete: Introduction
... The concrete (or specifically, the cement in it) needs moisture to hydrate and cure (harden). When concrete dries, it actually stops getting stronger. Concrete with too little water may be dry but is not fully reacted. The properties of such a concrete would be less than that of a wet concrete. The ...
... The concrete (or specifically, the cement in it) needs moisture to hydrate and cure (harden). When concrete dries, it actually stops getting stronger. Concrete with too little water may be dry but is not fully reacted. The properties of such a concrete would be less than that of a wet concrete. The ...
Mineral Tutorial Definition of a mineral: A mineral substance is a
... Definition of a mineral: A mineral substance is a naturally occurring solid that has been formed by geological processes, either on earth or in extraterrestrial bodies (Nickel 1995a). A mineral has a well-defined chemical composition and crystallographic properties, and which merits a unique name an ...
... Definition of a mineral: A mineral substance is a naturally occurring solid that has been formed by geological processes, either on earth or in extraterrestrial bodies (Nickel 1995a). A mineral has a well-defined chemical composition and crystallographic properties, and which merits a unique name an ...
PENTAL SODIUM (Thiopental sodium) and NORCURON
... Crystallographic projection along a axis was investigated and obtained which atoms are locating on these two planes. C6 and C7 atoms in the vecuronium bromide [4] were common atoms. It is clear that the other atoms were also locating on the same planes. But interactive effect of these carbon atoms w ...
... Crystallographic projection along a axis was investigated and obtained which atoms are locating on these two planes. C6 and C7 atoms in the vecuronium bromide [4] were common atoms. It is clear that the other atoms were also locating on the same planes. But interactive effect of these carbon atoms w ...
THE s -BLOCK ELEMENTS
... The s-block elements of the Periodic Table are those in which the last electron enters the outermost s-orbital. As the s-orbital can accommodate only two electrons, two groups (1 & 2) belong to the s-block of the Periodic Table. Group 1 of the Periodic Table consists of the elements: lithium, sodium ...
... The s-block elements of the Periodic Table are those in which the last electron enters the outermost s-orbital. As the s-orbital can accommodate only two electrons, two groups (1 & 2) belong to the s-block of the Periodic Table. Group 1 of the Periodic Table consists of the elements: lithium, sodium ...
Guess Paper – 2008 Class – X Subject
... (q) X, Y and Z are three crystalline solid soluble in water and have a common anion. To help you to identify X, Y and Z, following experimental observation are provided. Now complete the following : (i) A reddish-brown gas is obtained when X, Y and Z are warmed separately with concentrated sulphuric ...
... (q) X, Y and Z are three crystalline solid soluble in water and have a common anion. To help you to identify X, Y and Z, following experimental observation are provided. Now complete the following : (i) A reddish-brown gas is obtained when X, Y and Z are warmed separately with concentrated sulphuric ...
Crystallization of sulfides from alkali polysulfide fluxes
... coarse corundum powder in order to reduce the oxidation of the melt by air. During heating, excess sulfur evaporates and removes residual oxygen and water by reaction to S02 and H 2S, respectively, thus requiring the furnace to be placed in a hood with good ventilation. The temperature is set by a v ...
... coarse corundum powder in order to reduce the oxidation of the melt by air. During heating, excess sulfur evaporates and removes residual oxygen and water by reaction to S02 and H 2S, respectively, thus requiring the furnace to be placed in a hood with good ventilation. The temperature is set by a v ...
Encapsulated, Stabilized Alkali Metals for Birch
... metal. Sodium and sodium-potassium alloys in silica gel (Na-SG, Na2K-SG, and K2Na-SG) are free-flowing, non-pyrophoric solids that are easy to handle in the lab, pilot plant, and commercial manufacturing facility. They are typically produced with loadings of up to 40 wt. % alkali metal. The powders ...
... metal. Sodium and sodium-potassium alloys in silica gel (Na-SG, Na2K-SG, and K2Na-SG) are free-flowing, non-pyrophoric solids that are easy to handle in the lab, pilot plant, and commercial manufacturing facility. They are typically produced with loadings of up to 40 wt. % alkali metal. The powders ...
Classification of Minerals
... Substitution of Al3+ into tetrahedral sites allows additional elements Mg2+; Fe2+; Fe3+; Mn2+; Cr3+; Ti4+ to be put in octahedral sites (6-fold co-ordination) ...
... Substitution of Al3+ into tetrahedral sites allows additional elements Mg2+; Fe2+; Fe3+; Mn2+; Cr3+; Ti4+ to be put in octahedral sites (6-fold co-ordination) ...
Experiment 2
... adducts, LBH3. Adducts with thf, SMe2, pyridine and Me3N are widely used as mild organic oxidants, while BH3 is used as a protecting group for phosphines. The borohydride anion can also form complexes with transition metals, which may adopt a variety of structural types. It may act as an isolated a ...
... adducts, LBH3. Adducts with thf, SMe2, pyridine and Me3N are widely used as mild organic oxidants, while BH3 is used as a protecting group for phosphines. The borohydride anion can also form complexes with transition metals, which may adopt a variety of structural types. It may act as an isolated a ...
The s-block - WordPress.com
... the lattice enthalpy. Since lattice enthalpy decreases much more than the hydration enthalpy with increasing ionic size, the solubility increases as we go down the group. Problem: Why does the solubility of alkaline earth metal carbonates and sulphates in water decrease down the group? Solution The ...
... the lattice enthalpy. Since lattice enthalpy decreases much more than the hydration enthalpy with increasing ionic size, the solubility increases as we go down the group. Problem: Why does the solubility of alkaline earth metal carbonates and sulphates in water decrease down the group? Solution The ...
Cytochrome c Ab-2 (Clone 7H8.2C12)
... when handling this material. The chemical, physical, and toxicological properties of this material have not been thoroughly investigated. Appropriate measures should be taken to avoid skin and eye contact, inhalation, and ingestion. The material contains 0.09% sodium azide as a preservative. Althoug ...
... when handling this material. The chemical, physical, and toxicological properties of this material have not been thoroughly investigated. Appropriate measures should be taken to avoid skin and eye contact, inhalation, and ingestion. The material contains 0.09% sodium azide as a preservative. Althoug ...
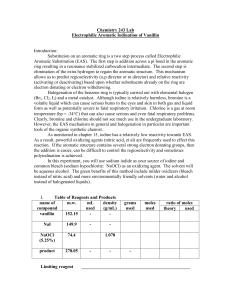
Electrophilic Aromatic Substitution of Vanillin
... The color should change from pale yellow to red-brown. 3. After the addition is complete, allow mixture to warm to room temperature and continue stirring another 10 minutes. Workup and Isolation: 4. Add 10 mL of sodium thiosulfate (10% w/w), then acidify with 10% HCl. Use litmus paper to monitor pH. ...
... The color should change from pale yellow to red-brown. 3. After the addition is complete, allow mixture to warm to room temperature and continue stirring another 10 minutes. Workup and Isolation: 4. Add 10 mL of sodium thiosulfate (10% w/w), then acidify with 10% HCl. Use litmus paper to monitor pH. ...
1 - AlvarezHChem
... 75.0mL of a 0.100M solution of AgNO3? (Hint: this involves stoichiometry and the molarity formula) ...
... 75.0mL of a 0.100M solution of AgNO3? (Hint: this involves stoichiometry and the molarity formula) ...
Sodium silicate

Sodium silicate is the common name for compounds with the formula Na2(SiO2)nO. A well-known member of this series is sodium metasilicate, Na2SiO3. Also known as waterglass or liquid glass, these materials are available in aqueous solution and in solid form. The pure compositions are colourless or white, but commercial samples are often greenish or blue owing to the presence of iron-containing impurities.They are used in cements, passive fire protection, textile and lumber processing, refractories, and automobiles. Sodium carbonate and silicon dioxide react when molten to form sodium silicate and carbon dioxide:Na2CO3 + SiO2 → Na2SiO3 + CO2Anhydrous sodium silicate contains a chain polymeric anion composed of corner-shared {SiO4} tetrahedral, and not a discrete SiO32− ion. In addition to the anhydrous form, there are hydrates with the formula Na2SiO3·nH2O (where n = 5, 6, 8, 9) which contain the discrete, approximately tetrahedral anion SiO2(OH)22− with water of hydration. For example, the commercially available sodium silicate pentahydrate Na2SiO3·5H2O is formulated as Na2SiO2(OH)2·4H2O and the nonahydrate Na2SiO3·9H2O is formulated as Na2SiO2(OH)2·8H2O.In industry, the various grades of sodium silicate are characterized by their SiO2:Na2O weight ratio (weight ratios can be converted to molar ratios by multiplication with 1.032), which can vary between 2:1 and 3.75:1. Grades with this ratio below 2.85:1 are termed alkaline. Those with a higher SiO2:Na2O ratio are described as neutral.