Chemistry-Unit-2-Battery-Technology-Cells-and-Battery
... hydrogen and oxygen produced during discharge back into water. Thus the battery retains its potency and requires no maintenance. Such batteries are sealed as there is no need to add water and this sealing prevents leakage of cell materials. www.bookspar.com | Website for Students | VTU NOTES | QUEST ...
... hydrogen and oxygen produced during discharge back into water. Thus the battery retains its potency and requires no maintenance. Such batteries are sealed as there is no need to add water and this sealing prevents leakage of cell materials. www.bookspar.com | Website for Students | VTU NOTES | QUEST ...
3rd Edition - Energy Storage and its Applications
... is stored chemically. A cell can be either primary (single-use) or secondary (rechargeable). The chemical energy of the cell contained in its active materials can be converted directly into electric energy by means of electrochemical oxidation-reduction (redox) reactions. The electrochemical cell ty ...
... is stored chemically. A cell can be either primary (single-use) or secondary (rechargeable). The chemical energy of the cell contained in its active materials can be converted directly into electric energy by means of electrochemical oxidation-reduction (redox) reactions. The electrochemical cell ty ...
Untitled - Johnson Matthey Battery Systems
... Lead-acid batteries are composed of a Lead-dioxide cathode, a sponge metallic Lead anode and a Sulphuric acid solution electrolyte. This heavy metal element makes them toxic and improper disposal can be hazardous to the environment. The cell voltage is 2 Volts. This chemistry is used in starter batt ...
... Lead-acid batteries are composed of a Lead-dioxide cathode, a sponge metallic Lead anode and a Sulphuric acid solution electrolyte. This heavy metal element makes them toxic and improper disposal can be hazardous to the environment. The cell voltage is 2 Volts. This chemistry is used in starter batt ...
battery technology - EngineeringDuniya.com
... combines the hydrogen and oxygen produced during discharge back into water. Thus the battery retains its potency and requires no maintenance. Such batteries are sealed as there is no need to add water and this sealing prevents leakage of cell materials. ...
... combines the hydrogen and oxygen produced during discharge back into water. Thus the battery retains its potency and requires no maintenance. Such batteries are sealed as there is no need to add water and this sealing prevents leakage of cell materials. ...
Article 4: The technological frontier of electrochemical energy storage
... transformation (a “phase change,” analogous to the evaporation and condensation that take a liquid to a gas and back to a liquid). Such phase changes erase all memory of the electrode’s mechanical history and allow a new cycle to start from scratch. The compromise here is that the ...
... transformation (a “phase change,” analogous to the evaporation and condensation that take a liquid to a gas and back to a liquid). Such phase changes erase all memory of the electrode’s mechanical history and allow a new cycle to start from scratch. The compromise here is that the ...
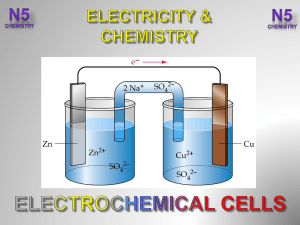
3.-Electrochemical-Cells-V2-
... The first small rechargeable battery produced for appliances, used a reaction between cadmium and a compound of nickel (nickel oxyhydroxide NiOOH) to produce a current. ...
... The first small rechargeable battery produced for appliances, used a reaction between cadmium and a compound of nickel (nickel oxyhydroxide NiOOH) to produce a current. ...
Cells and Batteries File
... Zinc – carbon dry cell (Leclanché cell) Alkaline dry cell Lithium – iodine cell Lead storage cell (car battery) Nickel – cadmium battery Nickel – metal hydride battery Lithium – ion battery Fuel cell ...
... Zinc – carbon dry cell (Leclanché cell) Alkaline dry cell Lithium – iodine cell Lead storage cell (car battery) Nickel – cadmium battery Nickel – metal hydride battery Lithium – ion battery Fuel cell ...
Answers for Review Questions Exam 3
... cell reaction reaches equilibrium, the battery is dead. Ex. Dry cell battery, Mercury, Lithium batteries, Alkaline Batteries. Problems: Can’t be reused, Dry battery- produces gaseous products, poor shelf life. Mercury Battery – poisonous. Secondary Battery – Uses a redox reaction, which is recharged ...
... cell reaction reaches equilibrium, the battery is dead. Ex. Dry cell battery, Mercury, Lithium batteries, Alkaline Batteries. Problems: Can’t be reused, Dry battery- produces gaseous products, poor shelf life. Mercury Battery – poisonous. Secondary Battery – Uses a redox reaction, which is recharged ...
Answers for Review Questions Exam 3
... cell reaction reaches equilibrium, the battery is dead. Ex. Dry cell battery, Mercury, Lithium batteries, Alkaline Batteries. Problems: Can’t be reused, Dry battery- produces gaseous products, poor shelf life. Mercury Battery – poisonous. Secondary Battery – Uses a redox reaction, which is recharged ...
... cell reaction reaches equilibrium, the battery is dead. Ex. Dry cell battery, Mercury, Lithium batteries, Alkaline Batteries. Problems: Can’t be reused, Dry battery- produces gaseous products, poor shelf life. Mercury Battery – poisonous. Secondary Battery – Uses a redox reaction, which is recharged ...
Lead–acid battery

The lead–acid battery was invented in 1859 by French physicist Gaston Planté and is the oldest type of rechargeable battery. Despite having a very low energy-to-weight ratio and a low energy-to-volume ratio, its ability to supply high surge currents means that the cells have a relatively large power-to-weight ratio. These features, along with their low cost, makes it attractive for use in motor vehicles to provide the high current required by automobile starter motors.As they are inexpensive compared to newer technologies, lead–acid batteries are widely used even when surge current is not important and other designs could provide higher energy densities. Large-format lead–acid designs are widely used for storage in backup power supplies in cell phone towers, high-availability settings like hospitals, and stand-alone power systems. For these roles, modified versions of the standard cell may be used to improve storage times and reduce maintenance requirements. Gel-cells and absorbed glass-mat batteries are common in these roles, collectively known as VRLA (valve-regulated lead–acid) batteries.Lead–acid battery sales account for 40–45% of the value from batteries sold worldwide (1999, not including China and Russia), a manufacturing market value of about US$15 billion.