A Biosensor Based on Immobilization of Horseradish Peroxidase in Chitosan Matrix Cross-linked with Glyoxal for Amperometric Determination of Hydrogen Peroxide
... copolymer of glucosamine and N-acetyglucosamine units linked by 1-4 glucosidic bonds, can be obtained by N-deacetylation of chitin, which is the second most abundant natural polymer [7]. Chitosan was selected as the matrix for immobilization of the enzyme because of an unusual combination of its pro ...
... copolymer of glucosamine and N-acetyglucosamine units linked by 1-4 glucosidic bonds, can be obtained by N-deacetylation of chitin, which is the second most abundant natural polymer [7]. Chitosan was selected as the matrix for immobilization of the enzyme because of an unusual combination of its pro ...
Development of Fungal Bioreactors for Water Related Treatment
... Wastewater, recycled irrigation water, and agricultural runoff can contain high levels of pathogenic bacteria, which pose a threat to human and ecosystem health. The use of a bioreactor containing mycelial mats of filamentous fungi is a novel treatment technology that incorporates physical, biologic ...
... Wastewater, recycled irrigation water, and agricultural runoff can contain high levels of pathogenic bacteria, which pose a threat to human and ecosystem health. The use of a bioreactor containing mycelial mats of filamentous fungi is a novel treatment technology that incorporates physical, biologic ...
Hydrogen: An Assessment of Its Potential for Energy Use
... safety, distribution infrastructure (e.g. refueling stations), and environmental pollution control in production of hydrogen and other elements of a hydrogen energy system. The major drawback to a hydrogen energy economy is the inherent inefficiency in the production of hydro ...
... safety, distribution infrastructure (e.g. refueling stations), and environmental pollution control in production of hydrogen and other elements of a hydrogen energy system. The major drawback to a hydrogen energy economy is the inherent inefficiency in the production of hydro ...
hydrogen storage
... cycle (Linde cycle). The gas is first compressed and then cooled in a heat exchanger, before it passes through a throttle valve where it undergoes an isenthalpic JouleThomson expansion, producing some liquid. The cooled gas is separated from the liquid and returned to the compressor via the heat exc ...
... cycle (Linde cycle). The gas is first compressed and then cooled in a heat exchanger, before it passes through a throttle valve where it undergoes an isenthalpic JouleThomson expansion, producing some liquid. The cooled gas is separated from the liquid and returned to the compressor via the heat exc ...
Disinfection and Sterilization
... • to clean surfaces that are "dirty," such as floors, sinks, and countertops; – a lower level of disinfectant – exception to this rule is: • a particular surface that is implicated in a nosocomial infection; – a bathroom contaminated with Clostridium difficile (spore-forming anaerobic bacterium) or ...
... • to clean surfaces that are "dirty," such as floors, sinks, and countertops; – a lower level of disinfectant – exception to this rule is: • a particular surface that is implicated in a nosocomial infection; – a bathroom contaminated with Clostridium difficile (spore-forming anaerobic bacterium) or ...
Hydrogen Sulfide in Nitrogen 0.0001% to 5.0%
... Stable under normal conditions. Conditions to Avoid: Avoid heat, flames, ignition sources and oxidizing agents. Incompatible Materials: Pure hydrogen sulfide is dangerously reactive with fuming or strong nitric acid and strong oxidizers; may ignite on contact with a variety of metal oxides (i.e.: co ...
... Stable under normal conditions. Conditions to Avoid: Avoid heat, flames, ignition sources and oxidizing agents. Incompatible Materials: Pure hydrogen sulfide is dangerously reactive with fuming or strong nitric acid and strong oxidizers; may ignite on contact with a variety of metal oxides (i.e.: co ...
Hydrogen Sulfide Control in Wastewater Collection Systems
... Hydrogen peroxide (H2O2) is applied to the wastewater system usually where there is a retention time of less than 5 hours and at least 30 minutes prior to the point where the hydrogen sulfide is released. Hydrogen peroxide is a stronger oxidant than either chlorine or potassium permanganate. Hydroge ...
... Hydrogen peroxide (H2O2) is applied to the wastewater system usually where there is a retention time of less than 5 hours and at least 30 minutes prior to the point where the hydrogen sulfide is released. Hydrogen peroxide is a stronger oxidant than either chlorine or potassium permanganate. Hydroge ...
Preparation and Properties of Hydrogen
... hydrogen will float. Because of the hydrogen molecule's small size, it will diffuse through many substances. Hydrogen gas is extremely flammable and will react with oxygen to form water with a release of a great deal of heat. The Hindenburg Zeppelin was destroyed in 1937 because of this reaction. He ...
... hydrogen will float. Because of the hydrogen molecule's small size, it will diffuse through many substances. Hydrogen gas is extremely flammable and will react with oxygen to form water with a release of a great deal of heat. The Hindenburg Zeppelin was destroyed in 1937 because of this reaction. He ...
Presentation - TOMI Environmental Solutions, Inc.
... Lamps deliver light either continuously or pulsed. B. Vaporized Hydrogen Peroxide; gaseous form, dehumidify ambient air and circulate vapour produced by generator throughout room usually high concentration of hydrogen peroxide or lower concentration mixed with silver ions. Certain technologies creat ...
... Lamps deliver light either continuously or pulsed. B. Vaporized Hydrogen Peroxide; gaseous form, dehumidify ambient air and circulate vapour produced by generator throughout room usually high concentration of hydrogen peroxide or lower concentration mixed with silver ions. Certain technologies creat ...
1 Assignment 5 Hydrogen – The Unique Element
... with water to produce hydrogen gas and a metal hydroxide. This means that they can be used as drying agents for solvents – the most commonly used in this regard is CaH2. p-Block molecular hydrides have different reactivities depending on their group and also the first row 2p compounds are significan ...
... with water to produce hydrogen gas and a metal hydroxide. This means that they can be used as drying agents for solvents – the most commonly used in this regard is CaH2. p-Block molecular hydrides have different reactivities depending on their group and also the first row 2p compounds are significan ...
1 Assignment 4 Hydrogen – The Unique Element
... with water to produce hydrogen gas and a metal hydroxide. This means that they can be used as drying agents for solvents – the most commonly used in this regard is CaH2. p-Block molecular hydrides have different reactivities depending on their group and also the first row 2p compounds are significan ...
... with water to produce hydrogen gas and a metal hydroxide. This means that they can be used as drying agents for solvents – the most commonly used in this regard is CaH2. p-Block molecular hydrides have different reactivities depending on their group and also the first row 2p compounds are significan ...
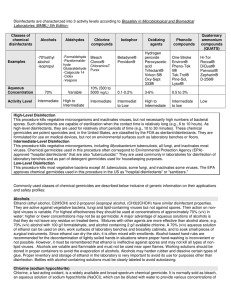
selection of a disinfectant
... available chlorine. Chlorine, especially as bleach, is highly alkaline and can be corrosive to metal. Its activity is considerably reduced by organic matter (protein). Storage of stock or working solutions of bleach in open containers, particularly at high temperatures, releases chlorine gas thus w ...
... available chlorine. Chlorine, especially as bleach, is highly alkaline and can be corrosive to metal. Its activity is considerably reduced by organic matter (protein). Storage of stock or working solutions of bleach in open containers, particularly at high temperatures, releases chlorine gas thus w ...
CHAPTER 9 HYDROGEN Position of Hydrogen in Periodic Table
... Properties of Hydrogen:Physical Properties:1) It is slightly soluble in water (about 2 %) 2) It is highly combustible and therefore should be handled carefully. 3) It lightest substance. The weight of one litre hydrogen at NTP is only 0.0899 g. Chemical properties:-Not very reactive due to high ...
... Properties of Hydrogen:Physical Properties:1) It is slightly soluble in water (about 2 %) 2) It is highly combustible and therefore should be handled carefully. 3) It lightest substance. The weight of one litre hydrogen at NTP is only 0.0899 g. Chemical properties:-Not very reactive due to high ...
UN1001: Section 11: Hydrogen Effects
... Hydride-forming metals are susceptible to H- embrittlement . . .e.g., Zr-alloy pressure tubes (in CANDUs) and fuel sheathing (in all water- cooled reactors) pick up hydrogen (or deuterium in heavy water ) by general corrosion. The hydrogen (D) migrates through the metal lattice to cool regions and t ...
... Hydride-forming metals are susceptible to H- embrittlement . . .e.g., Zr-alloy pressure tubes (in CANDUs) and fuel sheathing (in all water- cooled reactors) pick up hydrogen (or deuterium in heavy water ) by general corrosion. The hydrogen (D) migrates through the metal lattice to cool regions and t ...
H2O - WCCUSD.net
... 1. Warm up ≈50mL of water in a beaker to activate the yeast. The water should be ≈100°F, or about what comes out of the hot water tap. 2. Pour in ≈5mL (or ≈1 tsp, or ½ ...
... 1. Warm up ≈50mL of water in a beaker to activate the yeast. The water should be ≈100°F, or about what comes out of the hot water tap. 2. Pour in ≈5mL (or ≈1 tsp, or ½ ...
Hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula H2O2. In its pure form it is a colourless liquid, slightly more viscous than water; however, for safety reasons it is normally used as an aqueous solution. Hydrogen peroxide is the simplest peroxide (a compound with an oxygen-oxygen single bond) and finds use as a strong oxidizer, bleaching agent and disinfectant. Concentrated hydrogen peroxide, or 'high-test peroxide,' is a reactive oxygen species and has been used as a propellant in rocketry.Hydrogen peroxide is often described as being “water but with one more oxygen atom”, a description which can give the incorrect impression that there is a great deal of similarity between the two compounds. Pure hydrogen peroxide will explode if heated to boiling, will cause serious contact burns to the skin and can set materials alight on contact. For these reasons it is usually handled as a dilute solution (household grades are typically 3-6%). Its chemistry is dominated by the nature of its unstable peroxide bond.