Bonding - Department of Chemistry
... (i) No electrons are ejected, regardless of the intensity of the radiation, unless its frequency exceeds a threshold value characteristic of the metal. (ii) The kinetic energy of the electron increases linearly with the frequency of the incident radiation but is independent of the intensity of the ...
... (i) No electrons are ejected, regardless of the intensity of the radiation, unless its frequency exceeds a threshold value characteristic of the metal. (ii) The kinetic energy of the electron increases linearly with the frequency of the incident radiation but is independent of the intensity of the ...
Quantum Mechanics and Atomic Structure
... That’s what people tried to do at the beginning of the 20th century. We will describe how it was discovered that treating atomic constituents as exhibiting solely “particle-like behavior” inevitably lead to problems: inconsistencies and incorrect predictions. In fact, in order to begin to understand ...
... That’s what people tried to do at the beginning of the 20th century. We will describe how it was discovered that treating atomic constituents as exhibiting solely “particle-like behavior” inevitably lead to problems: inconsistencies and incorrect predictions. In fact, in order to begin to understand ...
LOW ENERGY NUCLEAR FUSION REACTIONS: QUANTUM
... InS ecf IOn 3' various results from the standard equatiOn ...
... InS ecf IOn 3' various results from the standard equatiOn ...
From electrons to quarks – the development of Particle Physics
... “Plum pudding or raisin cake model” atom = sphere of positive charge (diameter ≈10-10 m), with electrons embedded in it, evenly distributed (like raisins in cake) i.e. electrons are part of atom, can be kicked out of it – atom no more indivisible! ...
... “Plum pudding or raisin cake model” atom = sphere of positive charge (diameter ≈10-10 m), with electrons embedded in it, evenly distributed (like raisins in cake) i.e. electrons are part of atom, can be kicked out of it – atom no more indivisible! ...
LC Atomic Structure [PDF Document]
... • large no. not deflected - essentially empty space • many deflected at small angles ...
... • large no. not deflected - essentially empty space • many deflected at small angles ...
Note 14 - UF Physics
... (*) Neutrons with a mass equal to the proton mass, on the other hand, need only 5 MeV to generate 5 MeV protons... (*) The energy of neutrons was coming out just right from mass defect calculations: α+Be9 Æ C13 + n: ε=5 MeV! (*) The energy transmitted to heavier elements was also in a good agreement ...
... (*) Neutrons with a mass equal to the proton mass, on the other hand, need only 5 MeV to generate 5 MeV protons... (*) The energy of neutrons was coming out just right from mass defect calculations: α+Be9 Æ C13 + n: ε=5 MeV! (*) The energy transmitted to heavier elements was also in a good agreement ...
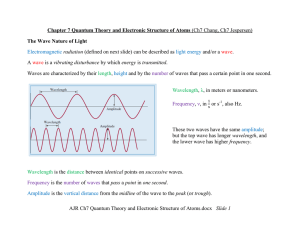
AJR Ch7 Quantum Theory and Electronic Structure of Atoms.docx
... According to classical electromagnetic (wave) theory, this effect can be attributed to the transfer of energy from the light to an electron. An alteration in either the intensity or wavelength of light would induce changes in the rate of emission of electrons from the metal. Furthermore, according ...
... According to classical electromagnetic (wave) theory, this effect can be attributed to the transfer of energy from the light to an electron. An alteration in either the intensity or wavelength of light would induce changes in the rate of emission of electrons from the metal. Furthermore, according ...
PPT
... Black side is hotter: gas molecules bounce off it with more momentum than on shiny side-this is a bigger effect than the photon momentum ...
... Black side is hotter: gas molecules bounce off it with more momentum than on shiny side-this is a bigger effect than the photon momentum ...
Chapter 19 - eLisa UGM
... (light), one must assume that light can be described both as wave (Interference, Diffraction) and particles (Photoelectric Effect, Frank-Hertz Experiment, x-ray production, x-ray scattering from electron) • To observe wave properties must make observations using devices with dimensions comparable to ...
... (light), one must assume that light can be described both as wave (Interference, Diffraction) and particles (Photoelectric Effect, Frank-Hertz Experiment, x-ray production, x-ray scattering from electron) • To observe wave properties must make observations using devices with dimensions comparable to ...
Chapter 5 Review Answer Key
... physically or combine chemically in simple, whole number ratios, and 4) that when reacting chemically, atoms can separate, join together, or rearrange, but atoms of one type never change into atoms of another. Thompson put gas into a glass tube at a near-vacuum and put a charge through it, causing a ...
... physically or combine chemically in simple, whole number ratios, and 4) that when reacting chemically, atoms can separate, join together, or rearrange, but atoms of one type never change into atoms of another. Thompson put gas into a glass tube at a near-vacuum and put a charge through it, causing a ...











![LC Atomic Structure [PDF Document]](http://s1.studyres.com/store/data/003605537_1-38ee6ce36c748f8bb00c7b0a30a77f99-300x300.png)