Symbol
... 19. Mrs. Stabile is terrible a darts. All of her darts hit the wall outside of the dartboard in a small cluster. a. Is Mrs. Stabile accurate when she plays darts? b. Is Mrs. Stabile precise when she plays darts? 20. In lab, you find the density of an object to be 5.16 g/mL. The actual density is 4.7 ...
... 19. Mrs. Stabile is terrible a darts. All of her darts hit the wall outside of the dartboard in a small cluster. a. Is Mrs. Stabile accurate when she plays darts? b. Is Mrs. Stabile precise when she plays darts? 20. In lab, you find the density of an object to be 5.16 g/mL. The actual density is 4.7 ...
From Gravitons to Galaxies (A New View of the Universe)
... I now quote him: “That one body may act upon another at a distance through a vacuum, without mediation of anything else, by and through which their action and force may be conveyed from one to the other, is to me so great an absurdity, that I believe no man who has in philosophical matters a compete ...
... I now quote him: “That one body may act upon another at a distance through a vacuum, without mediation of anything else, by and through which their action and force may be conveyed from one to the other, is to me so great an absurdity, that I believe no man who has in philosophical matters a compete ...
FirstSemesterReviewHonors
... 4. Be able to do conversion problems between moles and liters of a gas at STP. Example: What is the number of moles in 500 L of He gas at STP? 5. Be able to do conversion problems between atoms and moles. Example: How many moles of tungsten atoms are in 4.8 x 1025 atoms of tungsten? 6. Know how to c ...
... 4. Be able to do conversion problems between moles and liters of a gas at STP. Example: What is the number of moles in 500 L of He gas at STP? 5. Be able to do conversion problems between atoms and moles. Example: How many moles of tungsten atoms are in 4.8 x 1025 atoms of tungsten? 6. Know how to c ...
Question paper - Edexcel
... the curvature is perpendicular to the magnetic field. B the tracks curve in different directions. C the tracks have different curvatures. D there is no track before point P. (Total for Question 7 = 1 mark) 8 A racing car of mass 1200 kg travels at 0.63 rad s–1 around a bend of radius 50 m. ...
... the curvature is perpendicular to the magnetic field. B the tracks curve in different directions. C the tracks have different curvatures. D there is no track before point P. (Total for Question 7 = 1 mark) 8 A racing car of mass 1200 kg travels at 0.63 rad s–1 around a bend of radius 50 m. ...
Option 212: UNIT 2 Elementary Particles - X
... • No isolated quark has ever been observed • Believed impossible to obtain an isolated quark • If the PE between quarks increases with separation distance, an infinite amount of energy may be required to separate them • When a large amount of energy is added to a quark system, like a nucleon, a ...
... • No isolated quark has ever been observed • Believed impossible to obtain an isolated quark • If the PE between quarks increases with separation distance, an infinite amount of energy may be required to separate them • When a large amount of energy is added to a quark system, like a nucleon, a ...
Ch. 22 (Electrostatics)
... ⇒ Every atom is composed of a positively charged nucleus surrounded by negatively charged electrons. ⇒ The electrons, of all atoms are identical; they have the same mass and the same charge (also true of protons and neutrons) ⇒ Protons have the same charge as electron (but opposite signs) but have a ...
... ⇒ Every atom is composed of a positively charged nucleus surrounded by negatively charged electrons. ⇒ The electrons, of all atoms are identical; they have the same mass and the same charge (also true of protons and neutrons) ⇒ Protons have the same charge as electron (but opposite signs) but have a ...
50 Frequently Forgotten Facts Answer Key
... 4) Natural Decay: Parent Nuclide Decay particle + daughter nuclide [Tables N and O] a) Write the decay for U-238: _______ 23892U 42He + 23490Th_________ when the atomic # changes, the ID of the element changes as well. b) Write the decay for K-37:________ 3719K 0+1e + 3718Ar ____________ c) Wr ...
... 4) Natural Decay: Parent Nuclide Decay particle + daughter nuclide [Tables N and O] a) Write the decay for U-238: _______ 23892U 42He + 23490Th_________ when the atomic # changes, the ID of the element changes as well. b) Write the decay for K-37:________ 3719K 0+1e + 3718Ar ____________ c) Wr ...
chapter2
... represents an isotope of nickel that contains 28 protons and 32 neutrons in the nucleus. • Isotopes are also represented by the notation: Name-A, where Name is the name of the element and A is the mass number of the isotope. • An example of this isotope notation is magnesium-26. This represents an i ...
... represents an isotope of nickel that contains 28 protons and 32 neutrons in the nucleus. • Isotopes are also represented by the notation: Name-A, where Name is the name of the element and A is the mass number of the isotope. • An example of this isotope notation is magnesium-26. This represents an i ...
©FBC/London/Lisk/24thFeb2013 ELECTRON ARRANGEMENTS IN
... attraction will be less than that of the nucleus itself due to the presence of the first electron. Therefore, the value of, σ, is greater than zero. A value of zero would belie the existence of the charge, itself. If the value of the shielding factor were unity, the effective nuclear charge presente ...
... attraction will be less than that of the nucleus itself due to the presence of the first electron. Therefore, the value of, σ, is greater than zero. A value of zero would belie the existence of the charge, itself. If the value of the shielding factor were unity, the effective nuclear charge presente ...
02_Lecture
... 2.6 Radioactivity and Radioisotopes • When energy is given off spontaneously from the nucleus of an atom, it is called nuclear radiation. • Radiation comes in many different types and forms. Cosmic radiation is a natural radiation. It is a major source of radiation to which humans are exposed. • Mi ...
... 2.6 Radioactivity and Radioisotopes • When energy is given off spontaneously from the nucleus of an atom, it is called nuclear radiation. • Radiation comes in many different types and forms. Cosmic radiation is a natural radiation. It is a major source of radiation to which humans are exposed. • Mi ...
Dalton`s Laws worksheet
... Dalton’s Atomic Theory of Matter 1. Which of the following statements is part of Dalton’s atomic theory of matter? a. All atoms are identical b. All atoms of a given element are identical c. All atoms differ from one another d. Atoms of the same element can have a different shape 2. Dalton suggested ...
... Dalton’s Atomic Theory of Matter 1. Which of the following statements is part of Dalton’s atomic theory of matter? a. All atoms are identical b. All atoms of a given element are identical c. All atoms differ from one another d. Atoms of the same element can have a different shape 2. Dalton suggested ...
Matter Matters
... mostly floating around but not like gases where on the other hand of the gases, the gases are very wide apart from each other and they’re just roaming freely ...
... mostly floating around but not like gases where on the other hand of the gases, the gases are very wide apart from each other and they’re just roaming freely ...
Atomic Precision Tests and Light Scalar Couplings
... This gives a shift to the 1s-2s difference depending on the type of atom. Moreover this is significantly larger for muons compared to electrons: ...
... This gives a shift to the 1s-2s difference depending on the type of atom. Moreover this is significantly larger for muons compared to electrons: ...
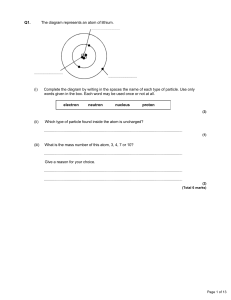
Atomic nucleus
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.