Principles of Statistical Mechanics and the
... essentially independent of the initial microstate. It is such an equilibrium that is described by the Boltzmann distribution. However, if the interactions between particles are very strong, then the forces on the particles are significantly different from those on the isolated particles we have so f ...
... essentially independent of the initial microstate. It is such an equilibrium that is described by the Boltzmann distribution. However, if the interactions between particles are very strong, then the forces on the particles are significantly different from those on the isolated particles we have so f ...
SU(3) - Physics
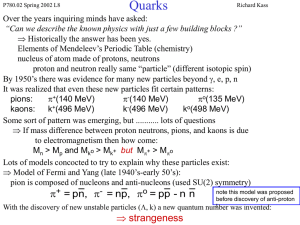
... Leptonic Decays of Vector Mesons What is the experimental evidence that quarks have non-integer charge ? Both the mass splitting of baryons and mesons and baryon magnetic moments depend on (e/m) not e. Some quark models with integer charge quarks (e.g. Han-Nambu) were also successful in explaining ...
... Leptonic Decays of Vector Mesons What is the experimental evidence that quarks have non-integer charge ? Both the mass splitting of baryons and mesons and baryon magnetic moments depend on (e/m) not e. Some quark models with integer charge quarks (e.g. Han-Nambu) were also successful in explaining ...
1 Introduction The periodic law discovered by Mendeleev in 1869
... It was shown that the Mills theory [10] can be derived from Lorentz quantum electrodynamics [11], obtained by combining the Lorentz classical electrodynamics and the Klein-Gordon equation. Consequently, the Mills theory does not contradict quantum mechanics, especially since it uses the same Pauli p ...
... It was shown that the Mills theory [10] can be derived from Lorentz quantum electrodynamics [11], obtained by combining the Lorentz classical electrodynamics and the Klein-Gordon equation. Consequently, the Mills theory does not contradict quantum mechanics, especially since it uses the same Pauli p ...
Hydroperoxide ion P.9 is much less basic than hydroxide ion P.10
... in each spin state on each atom, and the sum of the squares of all the c values on any one atom in all the molecular orbitals must also equal one. Thus the σ*-antibonding orbital of hydrogen will have c-values of 0.707 and –0.707, because these values make the whole set fit both criteria. ...
... in each spin state on each atom, and the sum of the squares of all the c values on any one atom in all the molecular orbitals must also equal one. Thus the σ*-antibonding orbital of hydrogen will have c-values of 0.707 and –0.707, because these values make the whole set fit both criteria. ...
Physics Today
... L = nħ, with n = 1, 2, 3, . . . . Introducing that quantum constraint into classical expressions, Bohr derived the radii ρn = (n2/Z)a0 and energies En = −(Z2/2n2)(e2/a0) of the allowed orbits for a oneelectron atom. The quantity a0 = ħ2/me2 was soon christened the Bohr radius; ħ = h/2π is the reduce ...
... L = nħ, with n = 1, 2, 3, . . . . Introducing that quantum constraint into classical expressions, Bohr derived the radii ρn = (n2/Z)a0 and energies En = −(Z2/2n2)(e2/a0) of the allowed orbits for a oneelectron atom. The quantity a0 = ħ2/me2 was soon christened the Bohr radius; ħ = h/2π is the reduce ...
Angular Impulse and Momentum for a Particle
... For a particle, the angular momentum is zero. We examine carefully the expression for the rate of change of angular momentum. Ḣ O = ṙ × mv + r × mv̇ . Since the coordinate system moves with the particle, both ṙ and v̇ are zero. This is true even if the coordinate system is not inertial, in contra ...
... For a particle, the angular momentum is zero. We examine carefully the expression for the rate of change of angular momentum. Ḣ O = ṙ × mv + r × mv̇ . Since the coordinate system moves with the particle, both ṙ and v̇ are zero. This is true even if the coordinate system is not inertial, in contra ...
Semiclassical theory of helium atom
... Figure 4 depicts, as a representative case, the level diagram of parahelium. The helium states and energy levels can be classified as follows: (i) the ground state and bound singly excited states, (ii) doubly excited resonant states, and (iii) unbound continuum states at energies above the two parti ...
... Figure 4 depicts, as a representative case, the level diagram of parahelium. The helium states and energy levels can be classified as follows: (i) the ground state and bound singly excited states, (ii) doubly excited resonant states, and (iii) unbound continuum states at energies above the two parti ...
Rubidium 85 D Line Data
... in K. This model is specified to have an accuracy better than ±5% from 298–550K. Older, and probably lessaccurate, sources of vapor-pressure data include Refs. [6] and [7]. The ionization limit is the minimum energy required to ionize a 85 Rb atom; this value is taken from Ref. [8]. The optical prop ...
... in K. This model is specified to have an accuracy better than ±5% from 298–550K. Older, and probably lessaccurate, sources of vapor-pressure data include Refs. [6] and [7]. The ionization limit is the minimum energy required to ionize a 85 Rb atom; this value is taken from Ref. [8]. The optical prop ...
study guide - Mechanical and Nuclear Engineering
... Describe and write the decay reactions for the various types of radioactive decay. List the physical quantities conserved in radioactive decay. Calculate the Q-value for any type of radioactive decay. Use the Q-value to quantify the kinetic energy of the decay products. Explain why the neutrino was ...
... Describe and write the decay reactions for the various types of radioactive decay. List the physical quantities conserved in radioactive decay. Calculate the Q-value for any type of radioactive decay. Use the Q-value to quantify the kinetic energy of the decay products. Explain why the neutrino was ...
Common Exam - 2004 Department of Physics University of Utah August 28, 2004
... L joined in a hinge at point “a”. The hinge is attached to a vertical wire and is free to slide up and down without friction. The far ends of the rods (points “b” and “c”) are attached to a common horizontal wire and are free to slide left and right without friction. The midpoints (centers of mass) ...
... L joined in a hinge at point “a”. The hinge is attached to a vertical wire and is free to slide up and down without friction. The far ends of the rods (points “b” and “c”) are attached to a common horizontal wire and are free to slide left and right without friction. The midpoints (centers of mass) ...
Alkali D Line Data
... in K. This model is specified to have an accuracy better than ±5% from 298–550K. Older, and probably lessaccurate, sources of vapor-pressure data include Refs. [6] and [7]. The ionization limit is the minimum energy required to ionize a 85 Rb atom; this value is taken from Ref. [8]. The optical prop ...
... in K. This model is specified to have an accuracy better than ±5% from 298–550K. Older, and probably lessaccurate, sources of vapor-pressure data include Refs. [6] and [7]. The ionization limit is the minimum energy required to ionize a 85 Rb atom; this value is taken from Ref. [8]. The optical prop ...
1 - GENCHEM
... 1. (a) This involves the photoelectric equation, E max = ½me v e 2 = hν - Φ, where Φ is the work function (7.29 x 10-19 J) representing the minimum energy (and hence maximum wavelength) of photons required for photoelectric effect. E min = 7.29 x 10-19 J = hc/λ max λ max = (6.626 x 10-34 J·s) (2.988 ...
... 1. (a) This involves the photoelectric equation, E max = ½me v e 2 = hν - Φ, where Φ is the work function (7.29 x 10-19 J) representing the minimum energy (and hence maximum wavelength) of photons required for photoelectric effect. E min = 7.29 x 10-19 J = hc/λ max λ max = (6.626 x 10-34 J·s) (2.988 ...
LHCb RICH detector
... Carbon fiber box with wings to stop photons radiated upstream of aerogel D263 glass filter to kill scattered photons and improve resolution Trial installation successful, aerogel goes in as last component in spring T. Bellunato- INSTR08 ...
... Carbon fiber box with wings to stop photons radiated upstream of aerogel D263 glass filter to kill scattered photons and improve resolution Trial installation successful, aerogel goes in as last component in spring T. Bellunato- INSTR08 ...
∑ ∑ ∑ - UCCS
... back with a horizontal velocity of 18 m/s. What is the change in momentum of the ball? ...
... back with a horizontal velocity of 18 m/s. What is the change in momentum of the ball? ...
Spin-Orbit Suppression of Cold Inelastic Collisions of Aluminum and Helium Please share
... spherical symmetry of the charge distribution, and fast for atoms in non-S states. However, it has recently been predicted that spin-orbit (SO) coupling in 2 P states can dramatically suppress those inelastic collisions [13], since in the 2 P1=2 state the precession of the orbital angular momentum l ...
... spherical symmetry of the charge distribution, and fast for atoms in non-S states. However, it has recently been predicted that spin-orbit (SO) coupling in 2 P states can dramatically suppress those inelastic collisions [13], since in the 2 P1=2 state the precession of the orbital angular momentum l ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.