Fractional charge in the fractional quantum hall system
... which imply 1/ν must be an odd integer, which is the simple fractional quantum Hall. As a summary, it is known that Laughlin wavefunction is the variational ground state of the FQHE. And it satisfies the equation of incompressible hydrodynamics equation. Thus, it is argued that the low energy excita ...
... which imply 1/ν must be an odd integer, which is the simple fractional quantum Hall. As a summary, it is known that Laughlin wavefunction is the variational ground state of the FQHE. And it satisfies the equation of incompressible hydrodynamics equation. Thus, it is argued that the low energy excita ...
Resonances in three-body systems S U L
... use interaction potentials of Coulombic or Gaussian forms. However, the formal and numerical approaches, used in this work, are in principle applicable to any three-body system. We are interested in how the energies and widths of resonances scale with the masses of the particles. In our calculations ...
... use interaction potentials of Coulombic or Gaussian forms. However, the formal and numerical approaches, used in this work, are in principle applicable to any three-body system. We are interested in how the energies and widths of resonances scale with the masses of the particles. In our calculations ...
Unit 1 Notes (general chem review)
... To make a mirror for a telescope, you coat the glass with a thin layer of aluminum to reflect the light. If the mirror has a diameter of 6.0 inches, and you want to have a coating that is 0.015 mm thick, how many grams of aluminum will you need? How many atoms of aluminum are in the coating? The den ...
... To make a mirror for a telescope, you coat the glass with a thin layer of aluminum to reflect the light. If the mirror has a diameter of 6.0 inches, and you want to have a coating that is 0.015 mm thick, how many grams of aluminum will you need? How many atoms of aluminum are in the coating? The den ...
File
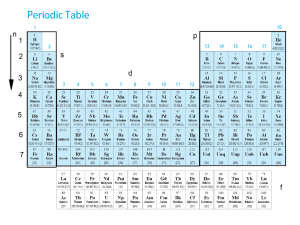
... Carbon is given a relative atomic mass (RAM) of 12. The RAM of other atoms compares them with carbon. Eg. Hydrogen has a mass of only one twelfth that of carbon and so has a RAM of 1. Below are the RAMs of some other elements. ...
... Carbon is given a relative atomic mass (RAM) of 12. The RAM of other atoms compares them with carbon. Eg. Hydrogen has a mass of only one twelfth that of carbon and so has a RAM of 1. Below are the RAMs of some other elements. ...
Optically polarized atoms
... • while nuclear size R is on the order of a few fermi (1 fermi = 1 fm = 10-13 cm) • Ratio between system size and wavelength similar to that for atoms • However, high-multipolarity transitions are often important; this is when low-multipolarity transitions are suppressed by selection rules – High-an ...
... • while nuclear size R is on the order of a few fermi (1 fermi = 1 fm = 10-13 cm) • Ratio between system size and wavelength similar to that for atoms • However, high-multipolarity transitions are often important; this is when low-multipolarity transitions are suppressed by selection rules – High-an ...
Chapter 3
... 33. An anion is defined as A. a charged atom or group of atoms with a net negative charge. B. a stable atom. C. a group of stable atoms. D. an atom or group of atoms with a net positive charge. 34. An cation is defined as A. a charged atom or group of atoms with a net negative charge. B. a stable a ...
... 33. An anion is defined as A. a charged atom or group of atoms with a net negative charge. B. a stable atom. C. a group of stable atoms. D. an atom or group of atoms with a net positive charge. 34. An cation is defined as A. a charged atom or group of atoms with a net negative charge. B. a stable a ...
01 introduction to quantum physics
... Each eigenfunction is associated with specific set of observables like energy, angular momentum, and spin. The eigenfunctions belong to a more general category of waves called wave functions that are just combinations of eigenfunctions. We have now to interpret the wavefunctions. ...
... Each eigenfunction is associated with specific set of observables like energy, angular momentum, and spin. The eigenfunctions belong to a more general category of waves called wave functions that are just combinations of eigenfunctions. We have now to interpret the wavefunctions. ...
Magnetic and Electric Flux Quanta: the Pion Mass
... ΦE2 2α Not only are the two values different and related by the fine structure constant, but in addition a factor of two has crept into the calculation. Thus far we have scrupulously avoided introducing any factors of two. The presence of a factor of two here is therefore thought to be a not-underst ...
... ΦE2 2α Not only are the two values different and related by the fine structure constant, but in addition a factor of two has crept into the calculation. Thus far we have scrupulously avoided introducing any factors of two. The presence of a factor of two here is therefore thought to be a not-underst ...
Effective electron-atom interactions and virial coefficients in alkali
... the plasma, i.e. from the Coulomb interaction between the elementary charged particles. The thermodynamic functions and the equation of state for a partially ionised plasma are usually derived from the effective interaction potentials between the different species -electrons, ions and neutral compos ...
... the plasma, i.e. from the Coulomb interaction between the elementary charged particles. The thermodynamic functions and the equation of state for a partially ionised plasma are usually derived from the effective interaction potentials between the different species -electrons, ions and neutral compos ...
CHAPTER TWO ATOMS, MOLECULES, AND IONS For Review 1. a
... b. All atoms of hydrogen have 1 proton in the nucleus. Different isotopes of hydrogen have 0, 1, or 2 neutrons in the nucleus. Because we are talking about atoms, this implies a neutral charge which dictates 1 electron present for all hydrogen atoms. If charged ions were included, then different ion ...
... b. All atoms of hydrogen have 1 proton in the nucleus. Different isotopes of hydrogen have 0, 1, or 2 neutrons in the nucleus. Because we are talking about atoms, this implies a neutral charge which dictates 1 electron present for all hydrogen atoms. If charged ions were included, then different ion ...
Chapter 40 Problems
... (b) What If? If photons are used, what minimum photon energy is needed to obtain the required resolution? 42. After learning about de Broglie’s hypothesis that particles of momentum p have wave characteristics with wavelength λ = h/p, an 80.0-kg student has grown concerned about being diffracted whe ...
... (b) What If? If photons are used, what minimum photon energy is needed to obtain the required resolution? 42. After learning about de Broglie’s hypothesis that particles of momentum p have wave characteristics with wavelength λ = h/p, an 80.0-kg student has grown concerned about being diffracted whe ...
Solid-state quantum computing using spectral holes M. S. Shahriar, P. R. Hemmer,
... systems called quantum bits or qubits must be able to address and manipulate individual qubits, to effect coherent interactions between pairs of qubits, and to read out the value of qubits 关1,2兴. Current methods for addressing qubits are divided into spatial methods, as when a laser beam is focused ...
... systems called quantum bits or qubits must be able to address and manipulate individual qubits, to effect coherent interactions between pairs of qubits, and to read out the value of qubits 关1,2兴. Current methods for addressing qubits are divided into spatial methods, as when a laser beam is focused ...
Packard Poster-2 - Northwestern University Mesoscopic Physics
... Cooper pairs of electrons are naturally created. Though the constituent electrons of these pairs form a single quantum object, they are spatially separated by a coherence length x which can extend several hundred nanometers. As this length scale is now easily accessible to modern nanolithographic te ...
... Cooper pairs of electrons are naturally created. Though the constituent electrons of these pairs form a single quantum object, they are spatially separated by a coherence length x which can extend several hundred nanometers. As this length scale is now easily accessible to modern nanolithographic te ...
hydrosulfuric
... The substance that is oxidized is the reducing agent The substance that is reduced is the oxidizing agent Chemists use oxidation numbers to account for the transfer of electrons in a RedOx reaction. ...
... The substance that is oxidized is the reducing agent The substance that is reduced is the oxidizing agent Chemists use oxidation numbers to account for the transfer of electrons in a RedOx reaction. ...
Momentum Transfer to a Free Floating Double Slit
... change of the scattered particle. Therefore, its quantum mechanical description will not provide new insights. In contrast, additional information is gained from the quantum mechanical description of the relative motion of the two scattering centers which involves a coherent superposition of double- ...
... change of the scattered particle. Therefore, its quantum mechanical description will not provide new insights. In contrast, additional information is gained from the quantum mechanical description of the relative motion of the two scattering centers which involves a coherent superposition of double- ...
Electron±electron correlations in carbon nanotubes
... When we compare the data of Fig. 1b with this model, striking deviations are observed. The transition lines in the data do not have identical slopes, even for a single CB region. Kinks and bends in the lines are frequent. The kinks in the CB region labelled n are particularly clear. Such a kink indi ...
... When we compare the data of Fig. 1b with this model, striking deviations are observed. The transition lines in the data do not have identical slopes, even for a single CB region. Kinks and bends in the lines are frequent. The kinks in the CB region labelled n are particularly clear. Such a kink indi ...
Multivalent Ionic Compounds
... For positive ions, one electron dot is removed from the valence shell for each positive charge of the ion. For negative ions, one electron dot is added to each valence shell for each negative charge of the ion. Square brackets are placed around each ion to indicate transfer of electrons ...
... For positive ions, one electron dot is removed from the valence shell for each positive charge of the ion. For negative ions, one electron dot is added to each valence shell for each negative charge of the ion. Square brackets are placed around each ion to indicate transfer of electrons ...
Chapter 2 - Molecular orbital theory
... De Broglie, 1924: Wave-particle duality describes electromagnetic radiation and matter (such as electrons, protons...) Schrodinger equation: Accounts for wave-particle duality, the motion of electrons in an atom, and quantized nature of atomic structure ...
... De Broglie, 1924: Wave-particle duality describes electromagnetic radiation and matter (such as electrons, protons...) Schrodinger equation: Accounts for wave-particle duality, the motion of electrons in an atom, and quantized nature of atomic structure ...
Physics 2170
... spin in every direction but experiments can only get the limited knowledge allowed by quantum mechanics. A better theory would allow one to get access to this information. This is called a hidden variable theory. In 1964, J.S Bell proved that local hidden variable theories would give a different res ...
... spin in every direction but experiments can only get the limited knowledge allowed by quantum mechanics. A better theory would allow one to get access to this information. This is called a hidden variable theory. In 1964, J.S Bell proved that local hidden variable theories would give a different res ...
Pauli Exclusion Principle
... J is quantized in space relative to the direction of B and the energy of the atomic state characterized by the angular momentum quantum number j is split into 2j + 1 energy levels corresponding to the 2j +1 possible values of the z component of J and therefore to the 2j +1 possible values of the z c ...
... J is quantized in space relative to the direction of B and the energy of the atomic state characterized by the angular momentum quantum number j is split into 2j + 1 energy levels corresponding to the 2j +1 possible values of the z component of J and therefore to the 2j +1 possible values of the z c ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.