Interactions and interference in quantum dots : kinks in
... positions may occur whenever the ground state of the dot is separated from an excited state with different spin by an energy of order ∆. The interference effects causing the separation are unique to each state and change upon tuning. In fact, the two states may switch at a certain point, the former ...
... positions may occur whenever the ground state of the dot is separated from an excited state with different spin by an energy of order ∆. The interference effects causing the separation are unique to each state and change upon tuning. In fact, the two states may switch at a certain point, the former ...
Interactions and Interference in Quantum Dots: Kinks in Coulomb
... positions may occur whenever the ground state of the dot is separated from an excited state with different spin by an energy of order ∆. The interference effects causing the separation are unique to each state and change upon tuning. In fact, the two states may switch at a certain point, the former ...
... positions may occur whenever the ground state of the dot is separated from an excited state with different spin by an energy of order ∆. The interference effects causing the separation are unique to each state and change upon tuning. In fact, the two states may switch at a certain point, the former ...
Standards Practice
... 11:1Students know chemical bonds between atoms in molecules such as Hz , CH4, NH3, HzCCHz , Nz, Clz, and many large biological molecules are covalent. 5. Which do not form covalent bonds? A. diatomic molecules B. large biological molecules C. molecules containing carbon D. salts 6. The bonds found i ...
... 11:1Students know chemical bonds between atoms in molecules such as Hz , CH4, NH3, HzCCHz , Nz, Clz, and many large biological molecules are covalent. 5. Which do not form covalent bonds? A. diatomic molecules B. large biological molecules C. molecules containing carbon D. salts 6. The bonds found i ...
Document
... The theoretical description of tunnel ionization of atoms in an alternating field has long attracted attention.' In a certain sense this task is more promising than that of ionization in the opposite multiphoton case. First, only the initial and final states of the electron are significant in tunnel ...
... The theoretical description of tunnel ionization of atoms in an alternating field has long attracted attention.' In a certain sense this task is more promising than that of ionization in the opposite multiphoton case. First, only the initial and final states of the electron are significant in tunnel ...
Basic Purpose of Quantum Mechanics
... simply an aspect of the processes of absorption and emission of radiation and had nothing to do with the physical reality of the radiation itself. In fact, he considered his quantum hypothesis a mathematical trick to get the right answer rather than a sizeable discovery. However, in 1905 Albert Eins ...
... simply an aspect of the processes of absorption and emission of radiation and had nothing to do with the physical reality of the radiation itself. In fact, he considered his quantum hypothesis a mathematical trick to get the right answer rather than a sizeable discovery. However, in 1905 Albert Eins ...
1A - The changing atom History of the atom • The model of the atom
... As the atom has the same number of protons and electrons it will have the same chemical properties. They are all hydrogen atoms because they all have the same number of protons Hydrogen can be used as an example:- ...
... As the atom has the same number of protons and electrons it will have the same chemical properties. They are all hydrogen atoms because they all have the same number of protons Hydrogen can be used as an example:- ...
Unit 4: Chemical Bonding Notes Chemical Bond—a mutual
... 1. Determine the total number of valence electrons in the atoms. 2. Arrange atoms to form a skeleton structure. If C is present, it is the central atom. Otherwise the least electronegative atom is ...
... 1. Determine the total number of valence electrons in the atoms. 2. Arrange atoms to form a skeleton structure. If C is present, it is the central atom. Otherwise the least electronegative atom is ...
Spectroscopy of electron ± electron scattering in a 2DEG
... compared with the 3D case (dashed line). ...
... compared with the 3D case (dashed line). ...
- Aboriginal Access to Engineering
... to how that structure is put together. In the center of the atom is a heavy nucleus; this is where the protons and neutrons are. Massless electrons orbit the nucleus. So a model of an atom, looks a bit like a model of the solar system. ...
... to how that structure is put together. In the center of the atom is a heavy nucleus; this is where the protons and neutrons are. Massless electrons orbit the nucleus. So a model of an atom, looks a bit like a model of the solar system. ...
Hybrid_Quantu_Classic_Dynamics!!
... • Mapping potential to drive reaction over barrier Vmap (r, R; m ) (1 - m )V11 (r, R ) mV22 (r, R ) • Thermodynamic integration to connect free energy curves • Peturbation formula to include adiabatic H quantum effects ...
... • Mapping potential to drive reaction over barrier Vmap (r, R; m ) (1 - m )V11 (r, R ) mV22 (r, R ) • Thermodynamic integration to connect free energy curves • Peturbation formula to include adiabatic H quantum effects ...
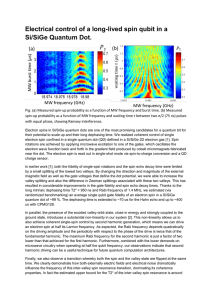
Electrical control of a long-lived spin qubit in a
... by a small splitting of the lowest two valleys. By changing the direction and magnitude of the external magnetic field as well as the gate voltages that define the dot potential, we were able to increase the valley splitting and also the difference in Zeeman splittings associated with these two vall ...
... by a small splitting of the lowest two valleys. By changing the direction and magnitude of the external magnetic field as well as the gate voltages that define the dot potential, we were able to increase the valley splitting and also the difference in Zeeman splittings associated with these two vall ...
Tunneling spectroscopy of disordered two
... second Hubbard band VC00 can be obtained by assuming a specific form for the ground state in a specific valley. Assuming, for simplicity, radial symmetry near the bottom of the valley, the Hartree energy between the two spins occupying this state, for a 2DEG well width of 23 nm (Ref. 5) is VC00 ≈ 3. ...
... second Hubbard band VC00 can be obtained by assuming a specific form for the ground state in a specific valley. Assuming, for simplicity, radial symmetry near the bottom of the valley, the Hartree energy between the two spins occupying this state, for a 2DEG well width of 23 nm (Ref. 5) is VC00 ≈ 3. ...
Energy dissipation of electron solitons in a quantum well
... The behavior of the system presented in Fig. 1共a兲 is a result obtained with the assumption of the ideal conductor, which immediately screens the external potential by formation of a proper surface charge. Let us calculate the induced potential for the dynamic response of the conducting medium on the ...
... The behavior of the system presented in Fig. 1共a兲 is a result obtained with the assumption of the ideal conductor, which immediately screens the external potential by formation of a proper surface charge. Let us calculate the induced potential for the dynamic response of the conducting medium on the ...
Note: This powerpoint is approximately 108 slides. The first... slides are my pitch to investors while the remaining slides...
... Another report was written by Dr. Henry Weinberg who was a professor of chemical engineering and chemistry at the University of California, Santa Barbara and he writes in his report: “To summarize, when first hearing of the claims of BLP it would be irrational not to be very skeptical, and prior to ...
... Another report was written by Dr. Henry Weinberg who was a professor of chemical engineering and chemistry at the University of California, Santa Barbara and he writes in his report: “To summarize, when first hearing of the claims of BLP it would be irrational not to be very skeptical, and prior to ...
Decay rates of planar helium - the Max Planck Institute for the
... approximation [22], Herrick’s algebraic approach [23], and the molecular adiabatic [24] approximation. These approaches make it possible to define some approximate good quantum numbers or classical constants of motion. The relative merits of these various descriptions depend on the states to be desc ...
... approximation [22], Herrick’s algebraic approach [23], and the molecular adiabatic [24] approximation. These approaches make it possible to define some approximate good quantum numbers or classical constants of motion. The relative merits of these various descriptions depend on the states to be desc ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.