atomic number
... are fast moving electrons. Since electrons are lighter than helium atoms, they are able to penetrate further, through several feet of air, or several millimeters of plastic or less of very light metals. ...
... are fast moving electrons. Since electrons are lighter than helium atoms, they are able to penetrate further, through several feet of air, or several millimeters of plastic or less of very light metals. ...
Scientific Papers
... other 50% is that it will not decay in the time allotted, so we would have absolutely no idea whether or not the cat would have died. Thus, we assume its matter was coexisting on two planes at once and neither living nor dying, but also both at the same time. “Albert Einstein was fond of asking, ‘Do ...
... other 50% is that it will not decay in the time allotted, so we would have absolutely no idea whether or not the cat would have died. Thus, we assume its matter was coexisting on two planes at once and neither living nor dying, but also both at the same time. “Albert Einstein was fond of asking, ‘Do ...
Document
... found in a particular region using statistical functions. Electron energy and position are complementary because ...
... found in a particular region using statistical functions. Electron energy and position are complementary because ...
NYS Regents Chemistry
... specific energy. The further the level is away from the nucleus the greater the energy of the electrons in it. 1. Bright line spectrum: When an electron in an atom gains just the right amount of energy, from an outside source, electron can shift to a higher energy state (excited state). However, the ...
... specific energy. The further the level is away from the nucleus the greater the energy of the electrons in it. 1. Bright line spectrum: When an electron in an atom gains just the right amount of energy, from an outside source, electron can shift to a higher energy state (excited state). However, the ...
New substances are formed by chemical reactions. When elements
... by covalent bonds. These bonds form when atoms share pairs of electrons. Chemical formulas The chemical formula of a compound shows how many of each type of atom join together to make the units which make up the compound. For example, in iron sulfide every iron atom is joined to one sulfur atom, so ...
... by covalent bonds. These bonds form when atoms share pairs of electrons. Chemical formulas The chemical formula of a compound shows how many of each type of atom join together to make the units which make up the compound. For example, in iron sulfide every iron atom is joined to one sulfur atom, so ...
Optical Properties of 1P State Electron Bubbles in
... mobility measurements and by investigations of the photon energies at which they absorb light [3]. At a critical negative pressure Pc of around -1.9 bars, a ground state bubble (1S) becomes unstable against expansion. Measurement [4] of this critical pressure gives good agreement with theoretical ca ...
... mobility measurements and by investigations of the photon energies at which they absorb light [3]. At a critical negative pressure Pc of around -1.9 bars, a ground state bubble (1S) becomes unstable against expansion. Measurement [4] of this critical pressure gives good agreement with theoretical ca ...
The Quantum Century
... demonstrated unequivocally that atoms themselves consist almost entirely of empty space, with negative particles (electrons) somehow circling a tiny, enormously dense nucleus - much like a miniature version of our solar system! Although it was very pleasing aesthetically that the world of the unimag ...
... demonstrated unequivocally that atoms themselves consist almost entirely of empty space, with negative particles (electrons) somehow circling a tiny, enormously dense nucleus - much like a miniature version of our solar system! Although it was very pleasing aesthetically that the world of the unimag ...
Quantum tomography of an electron - Hal-CEA
... T. Jullien1*, P. Roulleau1*, B. Roche1, A. Cavanna2, Y. Jin2 & D. C. Glattli1 ...
... T. Jullien1*, P. Roulleau1*, B. Roche1, A. Cavanna2, Y. Jin2 & D. C. Glattli1 ...
N5 Chemistry Summary notes 2017
... Take Hydrogen (H2) as an example. Each hydrogen atom has 1 positive proton in the nucleus and 1 negative electron in its electron shell. Two hydrogen atoms share their outer electron to obtain a full outer shell. ...
... Take Hydrogen (H2) as an example. Each hydrogen atom has 1 positive proton in the nucleus and 1 negative electron in its electron shell. Two hydrogen atoms share their outer electron to obtain a full outer shell. ...
Theoretical study of the phase evolution in a quantum dot in the
... Generalized Levinson’s theorem ...
... Generalized Levinson’s theorem ...
density becomes larger between the two nuclei. This re
... density becomes larger between the two nuclei. This results in an electrostatic attraction between the positive cores of the two atoms (for H2 these are the two protons) and the negative electron charge distribution between them. This effect is emphasized in the valence bond model used in chemistry. ...
... density becomes larger between the two nuclei. This results in an electrostatic attraction between the positive cores of the two atoms (for H2 these are the two protons) and the negative electron charge distribution between them. This effect is emphasized in the valence bond model used in chemistry. ...
Single-electron tunneling in the fractional quantum Hall effect regime∗
... The occupation numbers of subsequent occupied Landau levels thus have to form a strictly descending series. In Fig. 2 the triangular symbols are mean-field interaction energies for N = 5 and N = 6. The angular momentum values reached by the adiabatic mapping are dictated by Eqs. (25) and (27). For e ...
... The occupation numbers of subsequent occupied Landau levels thus have to form a strictly descending series. In Fig. 2 the triangular symbols are mean-field interaction energies for N = 5 and N = 6. The angular momentum values reached by the adiabatic mapping are dictated by Eqs. (25) and (27). For e ...
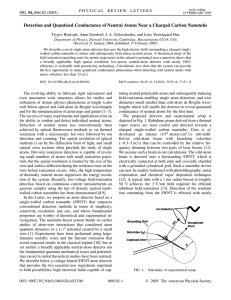
Detection and Quantized Conductance of Neutral Atoms Near a Charged... Trygve Ristroph, Anne Goodsell, J. A. Golovchenko, and Lene Vestergaard...
... The evolving ability to fabricate rigid micrometer and even nanometer scale structures allows for studies and utilization of atomic physics phenomena at length scales well below optical and cold-atom de Broglie wavelengths and for the miniaturization of atom traps and guides [1–7]. The success of ma ...
... The evolving ability to fabricate rigid micrometer and even nanometer scale structures allows for studies and utilization of atomic physics phenomena at length scales well below optical and cold-atom de Broglie wavelengths and for the miniaturization of atom traps and guides [1–7]. The success of ma ...
Reactions of Metals and Their Compounds
... Electrons that are in full shells. If we use the example of Na (sodium) , the electrons in the first shell ( 2 electrons) and the electrons in the second shell ( 8 electrons) are both found in full shells. These are the core electrons. • VALENCE electrons: The valence shell of the atom is the outerm ...
... Electrons that are in full shells. If we use the example of Na (sodium) , the electrons in the first shell ( 2 electrons) and the electrons in the second shell ( 8 electrons) are both found in full shells. These are the core electrons. • VALENCE electrons: The valence shell of the atom is the outerm ...
g - Porterville College Home
... ENGLISH - METRIC BRIDGE EXAMPLES Mass 1 lb = 453.6 g * 1 kg = 2.205 lb * ...
... ENGLISH - METRIC BRIDGE EXAMPLES Mass 1 lb = 453.6 g * 1 kg = 2.205 lb * ...
Communicating Research to the General Public
... responses to magnetic fields, to non-spectroscopic techniques like the di↵raction of light o↵ a crystal made of this protein, and even advanced computational techniques. My research as part of the Wright group seeks a new tool to add to this toolbox. There are a number of related problems that new t ...
... responses to magnetic fields, to non-spectroscopic techniques like the di↵raction of light o↵ a crystal made of this protein, and even advanced computational techniques. My research as part of the Wright group seeks a new tool to add to this toolbox. There are a number of related problems that new t ...
Chemistry SOL Review
... Quantum-Mechanical Model • Electron energy levels are wave functions. • Electrons are found in orbitals, regions of space where an electron is most likely to be found. • You can’t know both where the electron is and where it is going at the same time. • Electrons buzz around the nucleus like gnats b ...
... Quantum-Mechanical Model • Electron energy levels are wave functions. • Electrons are found in orbitals, regions of space where an electron is most likely to be found. • You can’t know both where the electron is and where it is going at the same time. • Electrons buzz around the nucleus like gnats b ...
Coherent transport through a quantum dot in a strong magnetic field *
... Whereas in the q"1 case the propagator has poles at all integer multiples of the noninteracting level spacing *e, in the interacting case the first q!1 poles (above the Fermi energy) are removed. This effect, which can be regarded as a remnant of the Coulomb blockade for particles with short-range i ...
... Whereas in the q"1 case the propagator has poles at all integer multiples of the noninteracting level spacing *e, in the interacting case the first q!1 poles (above the Fermi energy) are removed. This effect, which can be regarded as a remnant of the Coulomb blockade for particles with short-range i ...
Chemistry 3211 – Coordination Chemistry Part 4 Electronic Spectra
... −1, 0, +1) so is there a preference? Also, Hund only predicts ground states… what about higher energy excited states? Also, are the electrons paired or unpaired? Again, Hund says they should have parallel spins, but to have two electrons in different p orbitals with one being spin “up” and the other ...
... −1, 0, +1) so is there a preference? Also, Hund only predicts ground states… what about higher energy excited states? Also, are the electrons paired or unpaired? Again, Hund says they should have parallel spins, but to have two electrons in different p orbitals with one being spin “up” and the other ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.