* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Mass and Energy - Beverley High School
Survey
Document related concepts
Low-carbon economy wikipedia , lookup
Kinetic energy wikipedia , lookup
International Energy Agency wikipedia , lookup
Energy Charter Treaty wikipedia , lookup
Internal energy wikipedia , lookup
Energy returned on energy invested wikipedia , lookup
Negawatt power wikipedia , lookup
Mass–energy equivalence wikipedia , lookup
Energy policy of the European Union wikipedia , lookup
Nuclear fusion wikipedia , lookup
Energy Independence and Security Act of 2007 wikipedia , lookup
Energy applications of nanotechnology wikipedia , lookup
Energy efficiency in transport wikipedia , lookup
Transcript
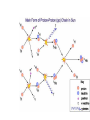
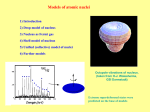
Mass and Energy The unified mass unit The mass number of a nuclide refers to the number of protons and nucleons in an atom 1H 7Li 238U 12C etc • Eg • However 1 hydrogen nucleus is only approximately 1/238 the mass of a uranium nucleus. This is because some of the mass is lost as “binding energy” when larger nuclei form. • Therefore there is the need to define a unit of mass with which we can compare the masses of nuclei. This unit of mass is called the unified mass unit. The unified mass unit The unified mass unit is defined as 1/12 of the mass of a 12C atom. (Iu = 1.661 x 10-27kg) The relative atomic mass (Ar) of any atom as a consequence of this is Mass of the atom ÷ 1/12 mass of 12C atom The mass energy relationship According to the special theory of relativity mass has an energy equivalent. Any change in mass corresponds to a change in energy according to the equation ΔE = c2Δm Correspondingly any change in energy corresponds to a change in mass The energy equivalent of u Iu = 1.661 x 10-27kg ΔE = c2Δm 1u = (2.998 x 108)2 x 1.661 x 10-27= 1.499 x10-9J Equivalently in eV 1.499 109 932 106 eV 19 1.602 10 i.e. 932MeV The unit MeV is often used as a measure of mass and this correctly emphasises the equivalence of mass and energy. Mass Defect and Binding Energy • The mass of a nucleus is always less than the total mass of its constituent nucleons. ( this is called mass defect) • This is because the formation of a nucleus involves the conversion of some of the mass of the constituents to energy. This is released as gamma rays. It is called binding energy. • To separate the components would require the input of that same amount of energy. The binding energy of a nucleus is the energy necessary to separate it into its component nucleons. Mass of neutrons 2 x 1.008 67u Mass of protons 2 x 1.007 28u Mass of He nucleus 4.002 60 u Mass defect = (2 x 1.008 + 2 x 1.007) – 4.002= 28.3MeV Binding energy per nucleon • The binding energy per nucleon of a nucleus is a measure of the stability of a nucleus.(This is the binding energy divided by the mass number.) • The larger the binding energy per nucleon, the more stable the nucleus. The nuclei at the top of the curve are most stable with the largest binding energy per nucleon. Those to the those nuclei to the left and right have less binding energy per nucleon Atoms with low numbers of nucleons can become more stable by fusing together to become larger nuclei and in the process releasing binding energy. Those to the right can achieve stability by becoming less massive by fission Fission A typical fissile material is uranium 235. This process occurs quite naturally in the material. If enough nuclei are present (ie the mass is above “critical mass”) the fusion process becomes an uncontrollable chain reaction. Fusion provides a much greater increase in binding energy per nucleon and therefore a much higher energy release per kg.