* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ch. 3 - Chemical Reactions
Fine chemical wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Biochemistry wikipedia , lookup
Hypervalent molecule wikipedia , lookup
California Green Chemistry Initiative wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Al-Shifa pharmaceutical factory wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Chemical weapon proliferation wikipedia , lookup
Isotopic labeling wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Process chemistry wikipedia , lookup
Electrochemistry wikipedia , lookup
Chemical weapon wikipedia , lookup
Safety data sheet wikipedia , lookup
Drug discovery wikipedia , lookup
George S. Hammond wikipedia , lookup
Chemical potential wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Chemical Corps wikipedia , lookup
Click chemistry wikipedia , lookup
Metalloprotein wikipedia , lookup
Chemical bond wikipedia , lookup
Chemical plant wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Chemical industry wikipedia , lookup
Molecular dynamics wikipedia , lookup
Chemical reaction wikipedia , lookup
History of chemistry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Transition state theory wikipedia , lookup
Rate equation wikipedia , lookup
VX (nerve agent) wikipedia , lookup
Atomic theory wikipedia , lookup
History of molecular theory wikipedia , lookup
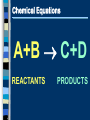
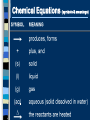
Chemical Reactions Intro to Reactions I II III IV V What is a Chemical Reaction A chemical reaction is a change in which one or more reactants change into one or more products. Bonds are broken and reformed What makes up a Chemical Reaction A chemical reaction is made up of chemical compounds, represented as chemical formula - Empirical Formula - Molecular Formula These compounds begin as reactants that then chemically change to become products. What are reactants & products A reactant is a substance present at the start of a reaction. A product is a substance present at the end of a reaction. Reactants and products are written as chemical equations for us to read and use. Chemical Equations A+B C+D REACTANTS PRODUCTS Chemical Equations (symbols & meanings) C. Johannesson Chemical Equations Coefficients and Subscripts Which is What •Subscript : the number located slightly below any element in a formula. Represents the ratio of numbers present in a molecule. H20 Subscript •Coefficient : Numbers placed in front of a formula to help balance an equation (related to moles) 2H20 Coefficient Signs of a Chemical Reaction Creation of heat and light Formation of a gas Formation of a precipitate Color change Writing Equations 2H2(g) + O2(g) 2H2O(g) Identify the substances involved. Use symbols to show: How many? - coefficient Of what? - chemical formula In what state? - physical state Practice Writing Equations Two atoms of aluminum react with three units of aqueous copper(II) chloride to produce three atoms of copper and two units of aqueous aluminum chloride. • How many? • Of what? • In what state? 2Al(s) + 3CuCl2(aq) 3Cu(s) + 2AlCl3(aq) Special Words Describing Equations Describing Coefficients: individual atom = “atom” covalent substance = “molecule” ionic substance = “unit” 3CO2 3 molecules of carbon dioxide 2Mg 2 atoms of magnesium 4MgO 4 unitsC. of magnesium oxide Johannesson Describing Equations Zn(s) + 2HCl(aq) ZnCl2(aq) + H2(g) • How many? • Of what? • In what state? One atom of solid zinc reacts with two molecules of aqueous hydrochloric acid to produce one unit of aqueous zinc chloride and one C. Johannesson gas. molecule of hydrogen Law of Conservation of Mass mass is neither created nor destroyed in a chemical reaction total mass stays the same atoms can only rearrange 4H 36 g 2O 4H 2O 4g 32 g C. Johannesson Law of Conservation of Mass Because of this law the number of atoms on the left side of the equation must ALWAYS equal the number of atoms on the right side of the equation. 4H 36 g 2O 4H 2O 4g 32 g C. Johannesson Balancing Equation Steps 1. Write the unbalanced equation. 2. Count atoms on each side. 3. Add coefficients to make #s equal. Coefficient subscript = # of atoms 4. Reduce coefficients to lowest possible ratio, if necessary. 5. Double check atom balance!!! Helpful Tips Balance one element at a time. Update ALL atom counts after adding a coefficient. If an element appears more than once per side, balance it last. Balance polyatomic ions as single units. “1 SO4” instead of “1 S” and “4 O” Balancing Example Aluminum and copper(II) chloride react to form copper and aluminum chloride. 2 Al + 3 CuCl2 3 Cu + 2 AlCl3 2 1 Al 1 2 3 1 Cu 1 3 6 2 Cl 3 6