* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Parkinson’s Disease Genetics
Genome (book) wikipedia , lookup
Tay–Sachs disease wikipedia , lookup
Public health genomics wikipedia , lookup
SNP genotyping wikipedia , lookup
Designer baby wikipedia , lookup
Dominance (genetics) wikipedia , lookup
Protein moonlighting wikipedia , lookup
Frameshift mutation wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Fetal origins hypothesis wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Gene nomenclature wikipedia , lookup
Microevolution wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Point mutation wikipedia , lookup
Neuronal ceroid lipofuscinosis wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
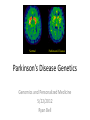
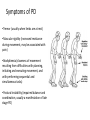
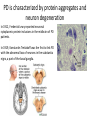
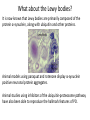
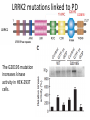
Parkinson’s Disease Genetics Genomics and Personalized Medicine 5/22/2012 Ryan Bell Parkinson’s disease (PD) PD is a chronic, progressive neurodegenerative disorder. PD afflicts 1.5 million people in North America, and over 4 million worldwide. In 1817, Dr. James Parkinson published the first widely acknowledged description of Parkinson’s disease: “An essay on the shaking palsy.” PD is primarily a sporadic disease affecting people over 60, but many familial forms have been identified, most which are early-onset. Symptoms of PD •Tremor (usually when limbs are at rest) •Muscular rigidity (increased resistance during movement, may be associated with pain) •Bradykinesia (slowness of movement resulting from difficulties with planning, initiating and executing movement, and with performing sequential and simultaneous tasks) •Postural instability (impaired balance and coordination, usually a manifestation of late stage PD) PD is characterized by protein aggregates and neuron degeneration In 1912, Frederick Lewy reported neuronal cytoplasmic protein inclusions in the midbrain of PD patients. In 1919, Konstantin Tretiakoff was the first to link PD with the abnormal loss of neurons in the substantia nigra, a part of the basal ganglia. Dopamine (DA) and PD In 1957, Arvid Carlsson and colleagues demonstrated that DA was an abundant neurotransmitter in the basal ganglia. They subsequently showed that feeding reserpine, which depletes DA in presynaptic neurons, to animals caused a loss of movement control similar to PD. They were able to alleviate these symptoms by administering L-DOPA, the precursor to DA which can cross the blood-brain barrier. Various forms of L-DOPA are still the most widely used treatment for the disease. Causes of PD? By the 1970s, it was clear that the classic, L-DOPA responsive symptoms of PD were a consequence of reduced DA levels resulting from DA neuron degeneration in the substantia nigra. The causes of the degeneration remained a mystery. Causes of PD? In 1982, a group of opiate addicts injected a dose of an illicitly produced batch of the synthetic opiate MPPP that was contaminated with a similar compound, MPTP. Within days, they exhibited permanent symptoms of severe end-stage PD, most notably akinesia, the inability to initiate movement. MPTP is a mitochondrial toxin MPTP was later shown to be selectively toxic to DA neurons because once it enters the brain, it is converted to MPP+, which has high affinity for the DA transporter used for DA reuptake in synapses. Inside DA neurons, MPP+ causes cell death by interfering with the electron transport chain in mitochondria, which decreases the ATP available to the cells, as well as causing oxidative stress by generating reactive oxygen species. Many pesticides are mitochondrial toxins The similarity of MPP+ to the widely used herbicide paraquat, as well as epidemiological data suggesting a positive correlation between pesticide exposure and incidence of PD led to many studies of the effects of pesticides on DA neurons in animal models. Paraquat and rotenone, a broad spectrum pesticide which is a mitochondrial toxin like MPP+, were eventually found to cause PD-like symptoms in animals. Rotenone What about the Lewy bodies? It is now known that Lewy bodies are primarily composed of the protein α-synuclein, along with ubiquitin and other proteins. Animal models using paraquat and rotenone display α-synuclein positive neuronal protein aggregates. Animal studies using inhibitors of the ubiquitin-proteosome pathway have also been able to reproduce the hallmark features of PD. α-synuclein • α-synuclein is highly abundant in neurons and associates with membranes and microtubules. It is thought to have activity similar to tau, which stabilizes microtubules, and is implicated in a number of neurodegenerative disorders, including Alzheimer's disease. • α-synuclein is also believed to be involved in vesicle trafficking. Is dopamine to blame? • Lewy bodies are found in other types of neurons, but only dopaminergic neurons die in large numbers in PD. • Under conditions of oxidative stress and formation of α-synuclein aggregates, storage of dopamine in synaptic vesicles can be disrupted, allowing the release of reactive metabolites into the cytosol, which further exacerbate oxidative stress. • Dopamine has also been shown to bind and stabilize α-synuclein protofibrils. • However, treatment with L-DOPA, which raises dopamine levels, has not been shown to accelerate disease progression. Genetic causes of PD Genes segregating with PD Proposed protein function Disease phenotype Autosomal recessive Parkin PINK1 DJ-1 Ubiquitin E3 ligase Mitochondrial kinase Oxidative stress protection Autosomal dominant UCH-L1 α-synuclein LRRK2 Ubiquitin hydrolase Early-onset PD Microtubule stabilization, vesicle trafficking Early-onset PD Kinase Late-onset PD Juvenile PD Early-onset PD Early-onset PD Of these inherited forms of PD, those caused by mutations in LRRK2 are the most common, and only they closely match the typical late-onset, as well as clinical and pathological features of sporadic PD. LRRK2 is also the most frequently mutated locus in cases of sporadic PD. LRRK2 (Leucine-rich repeat kinase 2) It is a member of the Roco protein family, which always possess Roc and COR domains in tandem. The Roc domain is a Ras like GTPase, while the COR domain functions as a hinge between the Ras and kinase domains. LRRK2 mutations linked to PD G2385R The G2019S mutation is dominant, and considered to be “disease causing,” but has incomplete penetrance. According to the International LRRK2 Consortium, the overall PD risk for carriers is 28% at age 59, 51% at age 69, and 74% at age 79, but other studies report lower odds. This mutation has only been observed in European, Ashkenazi Jewish, and Arab-Berber populations. LRRK2 mutations linked to PD G2385R The G2019S mutation increases kinase activity in HEK-293T cells. LRRK2 mutations linked to PD G2385R Among Asian populations, carriers of the G2385R mutation have a 3 fold increased risk for PD. The mutation is located in the WD40 domain of the protein, and has an unknown effect on its activity. PD Risk Loci SNP: i4000415 Gene name: GBA Risk Allele: C Odds ratio: 3.51 (European) P-value: 1.4E-14 This SNP lies in the GBA gene, which encodes glucocerebrosidase. Deficiency of this protein results in Gaucher’s disease, which leads to the accumulation of glucocerebrosides (fatty acids). The mechanism by which mutant form of the protein leads to PD is unknown, but is thought to involve aberrant handling and clearance of α-synuclein. Particularly relevant to Ashkenazi Jewish individuals, not observed in Asian Populations. PD Risk Loci SNP: rs356220 Gene name: SNCA Risk allele: T Odds ratio: 1.38 P-value: 3E-11 This SNP lies near the gene encoding α-synuclein. It likely contributes to PD risk via increased expression of the protein. PD Risk Loci SNP: rs393152 Gene name: MAPT (Microtubule associated protein tau) Risk allele: G Odds ratio: 1.30 (European) p-value: 2E-16 The tau protein has been implicated in a number of neurodegenerative diseases, termed tauopathies, including Alzheimer’s. Tau has been detected in Lewy bodies, suggesting a role in PD pathogenesis. This association was not replicated in a large study of Japanese individuals. PD Risk Loci SNP: rs947211 Gene name: SLC41A1/RAB7L1 Risk allele: G Odds ratio: 1.30 (European) P-value: 2E-12 SNP: rs823156 Gene name: SLC41A1 Risk allele: A Odds ratio: 1.2100 (Asian) P-value: 1E-7 SLC41A1 is a magnesium (Mg2+) transporter. Mg2+ deficiency is thought to be an environmental risk factor for PD. RAB7L1 is a small GTP-binding protein that plays an important role in regulation of exo- and endocytotic pathways. PD Risk Loci SNP: rs4698412 Gene name: BST1 Risk allele: A Odds ratio: 1.1400 P-value: 0.000002 This SNP is located near the BST1 gene, the product of which catalyzes formation of cyclic ADP-ribose, involved in calcium homeostasis. Sensitivity to disruption of calcium homeostasis has recently been proposed to be a cause of selective dopaminergic neuron vulnerability. PD Risk Loci SNP: rs11248060 Gene name: DGKQ Risk allele: T Odds ratio: 1.21 (European) P-value: 3E-12 This SNP is located within the DGKQ gene, which encodes a protein called diacylglycerol kinase theta. Its possible role in PD pathogenesis is uncharacterized. PD Risk Loci SNP: rs2390669 Gene name: STK39 Risk allele: C Odds ratio: 1.19 (European) P-value: 1.37E-9 This SNP is located within the STK39 gene, which encodes a protein kinase that is thought to be involved in the cellular response to oxidative stress. Risk reduction One of the early warning signs of PD is a reduction in the levels of glutathione (GSH), the major endogenous antioxidant. Dietary supplements of anti-oxidants may reduce risk for PD. Magnesium supplements may also be protective. Regular exercise has also been found to be associated with reduced incidence of the disease. Limiting exposure to pesticides would also decrease risk. Thanks for listening! Questions?