* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ch 3 Biochemistry Notes
Survey
Document related concepts
Protein–protein interaction wikipedia , lookup
Photosynthesis wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Puppy nutrition wikipedia , lookup
Introduction to genetics wikipedia , lookup
Chemical biology wikipedia , lookup
Genetic code wikipedia , lookup
Expanded genetic code wikipedia , lookup
Protein adsorption wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Biomolecular engineering wikipedia , lookup
History of molecular biology wikipedia , lookup
Abiogenesis wikipedia , lookup
Transcript
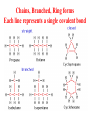
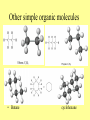
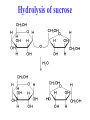
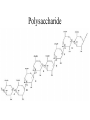
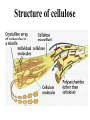
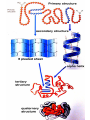
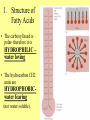
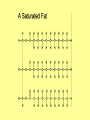
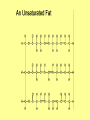
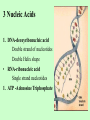
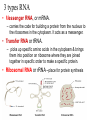
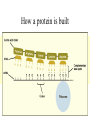
Chapter 3 Biochemistry Organic Molecules- The Building Blocks of Life I. What is an Organic Compound? • Contains carbon atoms that can be covalently bonded – to other carbon atoms – and to other atoms (like Hydrogen, Oxygen, Nitrogen, Sulfur, etc) • Carbon has 4 electrons in its outmost electron shell. How many sites does it have to form covalent bonds? 4 A. Carbon Bonding 4 sites Since it has to covalently bond to other atoms, a carbon atom works well as a basic building block – it combines in a variety of ways to form: 1. Chains- c-c-c-c 2. Branched Chain 3. Ring Forms c-c-c-c c-c c c c-c 4. single, double & triple bonds c-c c=c c=c Chains, Branched, Ring forms Each line represents a single covalent bond Methane is the simplest carbon compound1 Carbon & 4 hydrogen atoms Other simple organic molecules • Butane cyclohexane B. Functional groups See page 52 in your textbook for more on functional groups Example of adding a hydroxyl group – makes ethane into an alcohol- Example of adding an amino group - makes methane or ethane into- C. Sizes of Molecules 1. Monomers- small simple molecules 2. Polymers- complex molecules formed when monomers are bonded to each other 3. Reactions to build/ break down molecules • Condensation Reaction- when 2 monomers join together- a water is released (an H from 1 end and an OH from the other end are cut loose when the monomers join.) • Hydrolysis Reaction– when polymers are broken back down- they need a water added. Hydrolysis of sucrose D. Energy from ATP • Life needs a constant supply of energy • Chemical bonds store energy. • Food molecules are the $1,000 dollar bills of energy storage. They function as fuel molecules, storing large quantities of energy in a stable form over long periods of time. • One molecule that living things use to store energy is in the bonds of the ATP (Adenosine Triphosphate) molecule Adenosine Triphosphate Adenosine Triphosphate: Blue = ribose (a 5-carbon sugar) Green = adenine (a nirtogenous base) Yellow = phosphate groups Energy is stored in covalent bonds joining the phosphate groups (yellow). The ATP-ADP Cycle. Energy is needed for the formation of ATP and is released as the ATP is converted back to ADP and phosphate. III. 4 Classes of Organic Molecules • Built from carbon (C), hydrogen (H) and nitrogen (N)- in different ratios • Each group has distinct properties • Each organic molecule group has small molecules (monomers) that are linked to form a larger organic macromolecule (polymers) made of three to millions of monomer subunits. A. Carbohydrates - the most important energy source for cells • General formula [CH2O]n - where n is a number between 3 and 6. Ex- glucose= C6H12O6 • Carbohydrate functions – in short-term energy storage (such as sugar) – as intermediate-term energy storage (starch for plants and glycogen for animals) – as structural components in cells • (cellulose in the cell walls of plants and many protists) • chitin in the exoskeleton of insects and other arthropods. 1. Monosaccharides - single sugar units 2. Disaccharides - formed by linking two monosaccharides. 3. Polysaccharides - formed by linking many sugar units together • starch, glycogen, and cellulose are three common polysaccharides Polysaccharide Storage Polysaccharides 1. Starch - the storage carbohydrate in plants - formed by linking many glucose units using dehydration synthesis; – a – b 2. amylose - straight chain carbohydrate - up to 1000 glucose amylopectin - branches of 24-36 glucose off main Glycogen -storage carbohydrate in animals - glycogen is more extensively branched than amylopectin to increase the efficiency of storage. (Why?; glycogen is stored in liver and muscle; humans store enough glycogen for about 1 day; the levels of blood glucose and glycogen are controlled by insulin and glucagon. Insulin promotes the storage of glucose while glucagon promotes its release.) Structural Polysaccharides 1. Cellulose - the structural sugar of the plant cell wall; about 50% all organic carbon in biosphere is tied up in cellulose. Globally plants produce 1011 t cellulose per year 2. Chitin - the structural component in the exoskeleton of arthropods. It is also found in the fungal cell wall rather than cellulose as in plants. Structure of cellulose Humans consider cellulose to be roughage – why? Cellulose Fibers from Print Paper (SEM x1,080). This image is copyright Dennis Kunkel at www.DennisKunkel.com, used with permission. B. Proteins • Important in biological systems as control and structural elements. – Control functions carried out by enzymes and hormones. – Structural proteins function in the cell membrane, muscle tissue, etc. • The amino acid is the building block of proteins – an amino end (NH2) – a carboxyl end (COOH). – The R indicates the variable component (R-group) of each amino acid. • All living things (and even viruses) use various combinations of the same twenty amino acids. 1. Amino Acid *Amino acids are linked together by joining the amino end of one molecule to the carboxyl end of another. *Removal of water allows formation of a type of covalent bond known as a peptide bond. 2. PEPTIDE BONDS Some examples of proteins • Antibodies: they recognize molecules of invading organisms. • Receptors: part of the cell membrane, they recognize other proteins, or chemicals, and inform the cell... 'The Door Bell'. • Enzymes: assemble or digest. • Neurotransmitters and some hormones: Trigger the receptors... (the finger on the door bell...) • Channels, and pores: holes in the cell membrane (with or without a gate). Usually, filter the flow... Structure Of Proteins 1. Primary structure - the specific sequence of amino acids; 2. Secondary structure - H-bonds cause segments of the protein to be coiled or folded α-helix pleated sheet 3. Tertiary structure - results from interactions between amino acid side chains 4. Quaternary structure – multiple sub- groups Denaturation : Disrupting native (or natural) conformation; (if denaturation not too great the protein may return to its native conformation) Proteins can be denatured in several ways. 1. 2. 3. 4. 5. pH Salt heat different solvent chemical treatment 3. Enzymes • • • • Organic molecules that act as catalysts Essential to cell functions Most (not all) enzymes are proteins. Enzymes & substrates ( the reactant being catalyzed) fit together like a “lock & key” • Fit shapes reactants to weaken bonds so that less energy is needed for reaction. C. Lipids • Long-term energy storage. • Generally insoluble in polar substances such as water. • Secondary functions of lipids are as structural components – phospholipids are the major building block in cell membranes – "messengers" (hormones) play roles in communications within and between cells. 1. Structure of Fatty Acids • The carboxyl head is polar- therefore it is HYDROPHILIC – water loving • The hydrocarbon CH2 units are HYDROPHOBICwater fearing (not water soluble). Fatty acids • Can be saturated (meaning they have as many hydrogens bonded to their carbons as possible) • Unsaturated (with one or more double bonds connecting their carbons, hence fewer hydrogens). • A fat is solid at room temperature, while an oil is a liquid under the same conditions. The fatty acids in oils are mostly unsaturated, while those in fats are mostly saturated. 2. Triglycerides • Triglycerides are composed of three fatty acids (usually) covalently bonded to a 3carbon glycerol. Fats and oils function in energy storage. • Animals convert excess sugars into fats. • Most plants store excess sugars as starch, although some seeds and fruits have energy stored as oils (e.g. corn oil, peanut oil, palm oil, canola oil, and sunflower oil). – Fats yield 9.3 Kcal/gm, while carbohydrates yield 3.79 Kcal/gm. Fats store six times as much energy as glycogen. Diets & Fat Intake • Attempts to reduce the amount of fats present in specialized cells known as adipose cells that accumulate in certain areas of the human body. • By restricting the intakes of carbohydrates and fats, the body is forced to draw on its own stores to makeup the energy debt. • The body responds to this by lowering its metabolic rate, often resulting in a drop of "energy level." • Successful diets usually involve three things: decreasing the amounts of carbohydrates and fats; exercise; and behavior modification 3. Phospholipids • One fatty acid is replaced with a phosphate. • The negative charge(s) of the phosphate makes the “head” of the phospholipid hydrophilic. The long, hydrocarbon tail is non-polar and, therefore, hydrophobic. • *The water loving edge of the molecule orients toward water- the inside and outside of the cell. *The water fearing edges of the molecule orient toward each other to make a lipid “bilayer” - the construction of the cell membrane. 4. Cholesterol and steroids: • Structure is a lipid with 4 carbon rings with various functional groups attached • Cholesterol has many biological uses, such as its occurrence in the cell membranes, and its role in forming the sheath of some neurons. Excess cholesterol in the blood has been linked to atherosclerosis, hardening of the arteries. • Steroids are mainly used as hormones in living things •Structure of four steroids. Image from Purves et al., Life: The Science of Biology, 4th Edition, by Sinauer Associates (www.sinauer.com) and WH Freeman (www.whfreeman.com), used with permission. D. Nucleic Acids •Function - informational molecules – heredity/genetic, protein synthesis, and energy •A nucleotide is formed from a 5 carbon sugar, a phosphate and a nitrogen base. •Polymers formed by linking together long chains of nucleotide monomers. 3 Nucleic Acids 1. DNA-deoxyribonucleic acid Double strand of nucleotides Double Helix shape • RNA-ribonucleic acid Single strand nucleotides 1. ATP -Adenosine Triphosphate Structure of DNA double strand of nucleotides Structure of tRNA -single strand of nucleotides RNA differs from DNA in the following ways: • RNA is single stranded while DNA is double stranded. • RNA has a sugar called ribose while DNA has a sugar called deoxyribose. • RNA has the base uracil while DNA has the base thymine. How DNA & RNA work together • DNA(deoxyribonucleic acid) is the genetic material. • It functions by storing information regarding the sequence of amino acids in each of the body’s proteins. • This "list" of amino acid sequences is needed when proteins are synthesized. • Before protein can be synthesized, the instructions in DNA must first be copied to another type of nucleic acid called messenger RNA. • 3 types RNA • Messenger RNA, or mRNA. – carries the code for building a protein from the nucleus to the ribosomes in the cytoplasm. It acts as a messenger. • Transfer RNA or tRNA. – picks up specific amino acids in the cytoplasm & brings them into position on ribosome where they are joined together in specific order to make a specific protein. • Ribosomal RNA or rRNA –place for protein synthesis How a protein is built