* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture 26
Peptide synthesis wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Butyric acid wikipedia , lookup
Photosynthesis wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metalloprotein wikipedia , lookup
Microbial metabolism wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Electron transport chain wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Biosynthesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biochemistry wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
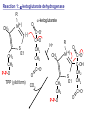
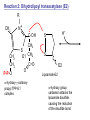
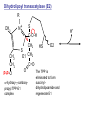
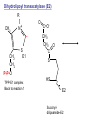
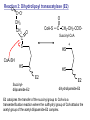
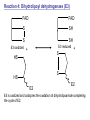
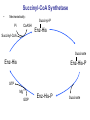
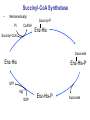
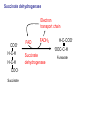
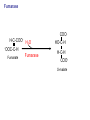
Lecture 26 – TCA cycle Page 766 Factors controlling the activity of the PDC. (b) Covalent modification in the eukaryotic complex. Page 781 Figure 21-17b Control by phosporylation/dephosphorylation • • • • Occurs only in eukaryotic complexes The E2 subunit has both a pyruvate dehydrogenase kinase and pyruvate dehydrogenase phosphatase that act to regulate the E1 subunit. Kinase inactivates the E1 subunit. Phosphatase activates the subunit. Ca2+ is an important secondary messenger, it enhances phosphatase activity. Page 766 Citric acid cycle: 8 enzymes • 1. 2. 3. 4. 5. 6. 7. 8. Oxidize an acetyl group to 2 CO2 molecules and generates 3 NADH, 1 FADH2, and 1 GTP. Citrate synthase: catalyzes the condensation of acetyl-CoA and oxaloacetate to yield citrate. Aconitase: isomerizes citrate to the easily oxidized isocitrate. Isocitrate dehydrogenase: oxidizes isocitrate to the -keto acid oxalosuccinate, coupled to NADH formation. Oxalosuccinate is then decarboxylated to form -ketoglutarate. (1st NADH and CO2). -ketoglutarate dehydrogenase: oxidatively decarboxylates ketoglutarate to succinyl-CoA. (2nd NADH and CO2). Succinyl-CoA synthetase converts succinyl-CoA to succinate. Forms GTP. Succinate dehydrogenase: catalyzes the oxidation of central single bond of succinate to a trans double bond, yielding fumarate and FADH2. Fumarase: catalyzes the hydration of the double bond to produce malate. Malate dehydrogenase: reforms OAA by oxidizing 2ndary OH group to ketone (3rd NADH) Citric acid cycle 3NAD+ + FAD + GDP + Pi + acetyl-CoA 3NADH + FADH2 + GTP + CoA + 2CO2 3NADH + FADH2 are oxidized by the electron transport chain and drive ATP synthesis. Citrate synthase: reaction 1 • • • Catalyzes the condensation of acetyl-CoA and oxaloacetate to form citrate. Oxaloacetate has to bind to the enzyme before acetyl-CoA. Oxaloacetate binds to the enzyme causing a conformational shift that opens the acetyl-CoA binding site. (induced fit) • Reaction mechanism is a mixed aldol-Claisen ester condensation (acid-base catalysis). • Acetyl forms an enol intermediate. • Three important amino acids: His274, Asp375, His320 1. The formation of enolate form of acetyl-CoA is the rate-limiting step. Asp375 acts as a general base to remove a proton from the methyl group of the acetyl-CoA. His 274 is hydrogen bonded to acetyl-CoA. 2. Citryl-CoA is formed in a second acid-base catalyzed reaction step. Acetyl-CoA enolate form attacks oxaloacetate. 3. Citryl-CoA is hydrolyzed to citrate and CoA. Stereospecific reactions (acetate onlly forms citrate’s pro-S carboxymethyl group. Page 783 Aconitase: reaction 2 • • • • • • Catalyzes the reversible isomerization of citrate and isocitrate with cis-aconitate as an intermediate. Citrate is prochiral so aconitase can distinguish between citrate’s pro-R and pro-S carboxymethyl groups. Has a covalently bound [4Fe-4S] iron-sulfur cluster. Fea atom coordinates with the OH group of citrate The iron-sulfur cluster does not perform a redox reaction but instead is able to stabilize the ligand-substrate complex. Second stage of the reaction rehydrates cis-aconitate’s double bond in a stereospecific trans addition to form only the 2R,3S isocitrate form. Page 784 Isocitrate dehydrogenase: reaction 3 • • • • • • • • • • Catalyzes the oxidiation of isocitrate to form aketoglutarate 1st reaction to produce NADH and CO2. Activated by AMP and ADP Inhibited by NADH and NADPH Competitively bind to the NAD+ binding site. Requires Mn2+ or Mg2+ cofactor. Mechanistically-oxidize to the b-keto acid. 2 forms of the enzyme Mitochondrial form is NAD+ dependant [ADP] E. coli, mitochondrial, cytoplasmic forms NADP+ dependant. Page 785 Figure 21-21 Probable reaction mechanism of isocitrate dehydrogenase. -ketoglutarte dehydrogenase complex • • • • • • Catalyzes the oxidiation and decarboxylation of ketoglutarate to produce succinyl-CoA. Consists of -ketoglutarte dehydrogenase (E1), dihydrolipoyl transsuccinylase (E2), and dihydrolipoyl dehydrogenase (E3). Mechanistically resembles PDC. 2nd reaction to produce NADH and CO2. 5 coenzymes (TPP, lipoic acid, CoA, FAD, NAD+) Product inhibition (Succinyl-CoA), NADH Reaction 1: -ketoglutarate dehydrogenase R -ketoglutarate O (+) N CH3 C O (-) C=O CH2 CH2 S E1 P-P-O TPP (ylid form) CH2 CH2 R H+ CH3 O N(+) C-O- C-OH CH2 C-OO CO2 CH2 CH2 P-P-O S E1 CH2 C-OO Reaction 2: Dihydrolipoyl transacetylase (E2) R CH3 N+ C-OH H+ S CH2 CH2 CH2 P-P-O S E1 CH2 S C-OO -hydroxy--carboxypropyl TPP-E1 complex E2 Lipoamide-E2 -hydroxy group carbanion attacks the lipoamide disulfide causing the reduction of the disulfide bond Dihydrolipoyl transacetylase (E2) R CH3 S N+ H+ C-O-H CH2 CH2 CH2 P-P-O S E1 CH2 HS E2 C-OO -hydroxy--carboxypropyl TPP-E1 complex The TPP is eliminated to form succinyl dihydrolipoamide and regenerate E1 Dihydrolipoyl transacetylase (E2) R CH3 O N+ - CH2 CH2 S E1 C-O- CH2 CH2 O C S P-P-O TPP-E1 complex Back to reaction 1 HS E2 Succinyldilipoamide-E2 Reaction transacetylase (E2) O 3: Dihydrolipoyl C-OO CH2 CoA-S C CH2-CH2-COOCH2 O Succinyl-CoA C + S HS CoA-SH HS HS E2 Succinyldilipoamide-E2 E2 dihydrolipamide-E2 E2 catalyzes the transfer of the succinyl group to CoA via a transesterification reaction where the sulfhydryl group of CoA attacks the acetyl group of the acetyl dilipoamide-E2 complex. Reaction 4: Dihydrolipoyl dehydrogenase (E3) FAD FAD S SH S SH E3 reduced + E3 oxidized + S HS S HS E2 E2 E3 is oxidized and catalyzes the oxidation of dihydrolipoamide completing the cycle of E2. Reaction 5: Dihydrolipoyl dehydrogenase (E3) FAD FADH2 FAD SH S S SH S S NAD+ NADH + H+ E3 oxidized E3 is oxidized by the enzyme bound FAD which is reduced to FADH2. This reduces NAD+ to produce NADH. Succinyl-CoA Synthetase • • • Hydrolyzes the “high-energy” succinyl-CoA with the coupled synthesis of a “high-energy” nucleoside triphosphate. In mammals, GTP In bacteria and plants, ATP. Succinyl-CoA Synthetase • Mechanistically: Succinyl-P Pi CoASH Enz-His Succinyl-CoA Succinate Enz-His Enz-His-P GTP Mg++ GDP Enz-His-P Succinate Succinyl-CoA Synthetase • Mechanistically: Pi CoASH Succinyl-P Enz-His Succinyl-CoA Succinate Enz-His Enz-His-P GTP Mg++ GDP Enz-His-P Succinate Page 787 Figure 21-22a Reactions catalyzed by succinyl-CoA synthetase. Formation of succinyl phosphate, a “high-energy” mixed anhydride. Page 787 Figure 21-22b Reactions catalyzed by succinyl-CoA synthetase. Formation of phosphoryl–His, a “high-energy” intermediate. Page 787 Figure 21-22c Reactions catalyzed by succinyl-CoA synthetase. Transfer of the phosphoryl group to GDP, forming GTP. Succinate dehydrogenase • • • • Only makes the trans-fumarate. Donates electrons directly into complex II of the respiratory chain (ubiquinone (Q)). If the respiratory chain is inhibited, FAD is unable to accept electrons and TCA cycle stops. Inhibited by OAA, activated by coenzyme Q (part of electron tranport chain). Page 787 Figure 21-23 Covalent attachment of FAD to a His residue of succinate dehydrogenase. Succinate dehydrogenase Electron transport chain COOH-C-H H-C-H COOSuccinate FAD FADH2 H-C-COO-OOC-C-H Succinate dehydrogenase Fumarate Succinate dehydrogenase • • • • • Catalyzes the stereospecific dehydrogenation of succinate to fumurate. Enzyme strongly inhibited by malonate (competitive inhibitor). Contains an FAD-electron acceptor. FAD functions to oxidize alkanes to alkenes (vs. NAD+ which oxidizes alcohols to aldehydes and ketones). FAD covalently linked to His from enzyme. Fumarase (fumarate hydratase) • • • Catalyzes the stereospecific dehydrogenation of succinate to fumurate. Only catalyzes the trans-fumarate Competitively inhibited by maleate (cis doublebond). Fumarase H-C-COO- H O 2 -OOC-C-H Fumarase Fumarate COOHO-C-H H-C-H COOS-malate Malate dehydrogenase • • • Catalyzes the final reaction of the citric acid cycle-regeneration of oxaloacetate. Oxidizes S-malate’s OH group to a ketone in an NAD+ dependent reaction. Produces NADH. COOHO-C-H H-C-H COOS-malate NAD+ NADH Malate dehydrogenase COOO=C-H H-C-H COOOxaloacetate Page 766 Total (PDH and TCA) NAD+ + pyruvate + CoA PDH NADH + acetyl-CoA + CO2 3NAD+ + FAD + GDP + Pi + TCA acetyl-CoA Pyruvate 4NAD+ FAD GDP + Pi 3CO2 4NADH FADH2 GTP 3NADH + FADH2 + GTP + CoA + 2CO2 NADH DH Complex II Nucleoside diphosphokinase 12ATP 2ATP 1ATP Page 791 Figure 21-25 Regulation of the citric acid cycle. Regulation of citric acid cycle • • • • • Rate-controlling enzymes: citrate synthase, isocitrate dehydrogenase, -ketoglutarate dehydrogenase. Regulated by substrate availability, product inhibition and inhibition by other cycle intermediates (generally simpler than glycolysis). Citrate synthase- inhibited by citrate, -KG, succ-CoA, NADH, activated by OAA and CoASH. Isocitrate dehydrogenase-Requires AMP/ADP Activated by Ca2+, inhibited by NADPH or NADH -ketoglutarate dehydrogenase-inhibited by Succ-CoA, NADH, ATP. Activated by Ca2+ Pyruvate dehydrogenase-inhibited by NADH and acetyl-CoA Page 793 Figure 21-26 Amphibolic functions of the citric acid cycle. Pathways that use citric acid cycle intermediates Reactions that utilize intermediates of TCA cycle are called cataplerotic reactions 1. Gluconeogenesis-in cytosol uses OAA. In the mitochondria uses malate (transported across the membrane). 2. Lipid biosynthesis-requires acetyl-CoA. Transported across the membrane by the breakdown of citrate. ATP + citrate + CoA 3. ADP + Pi + oxaloacetate + acetyl-CoA Amino acid biosynthesis-can use -ketoglutarate to form glutamic acid in a reductive amination reaction (uses NAD+ or NADP+ depending on enzyme) -ketoglutarate + NAD(P)H + NH4+ glutamate + NAD(P)+ + H2O Pathways that use citric acid cycle intermediates 3. Amino acid biosynthesis-can also use -ketoglutarate and oxaloacactate in transamination reactions -ketoglutarate + alanine oxaloacetate + alanine 4. 5. glutamate + pyruvate aspartate + pyruvate Porphyrin biosynthesis- utilizes succinyl-CoA Complete oxidation of amino acids - amino acids first converted to PE by PEPCK Pathways that make citric acid cycle intermediates Reactions that replenish intermediates of TCA cycle are called anaplerotic reactions Pyruvate carboxylase- produces oxaloacetate Pyruvate + CO2 + ATP + H2O oxaloacetate + ADP + Pi Degradative pathways generate TCA cycle intermediates 1. Oxidation of odd-chain fatty acids generates succinyl-CoA 2. Ile, Met, Val generate succinyl-CoA 3. Transamination and deamination of amino acids leads to ketoglutarate and oxaloacetate. Each NADH yields ≈ 3ATP Each FADH2 yields ≈ 2ATP Total yields ≈ 38ATP for each fully oxidized glucose. Page 851 Glyoxylate cycle • The glyoxylate cycle results in the net conversion of two acetyl-CoA to succinate instead of 4 CO2 in citric acid cycle. • Succinate is transferred to mitochondrion where it can be converted to OAA (TCA) • Can go to cytosol where it is converted to oxaloacetate for gluconeogenesis. Net reaction 2Ac-CoA + 2NAD+ + FAD OAA + 2CoA + 2NADH +FADH2 + 2H+ Plants are able to convert fatty acids to glucose through this pathway Page 798