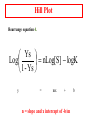* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Hemoglobin and Myoglobin
Survey
Document related concepts
Radical (chemistry) wikipedia , lookup
Photosynthesis wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Point mutation wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Drug design wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Oxygen toxicity wikipedia , lookup
Biochemistry wikipedia , lookup
Ligand binding assay wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Transcript
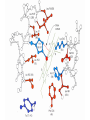
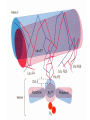
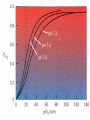
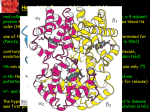
Protein Function Globins and Antibodies 3/10/2003 Hemoglobin and Myoglobin Because of its red color, the red blood pigment has been of interest since antiquity. •First protein to be crystallized - 1849. •First protein to have its mass accurately measured. •First protein to be studied by ultracentrifugation. •First protein to associated with a physiological condition. •First protein to show that a point mutation can cause problems. •First proteins to have X-ray structures determined. •Theories of cooperativity and control explain hemoglobin function The structure of myoglobin and hemoglobin Andrew Kendrew and Max Perutz solved the structure of these molecules in 1959 to 1968. The questions asked are basic. What chemistry is responsible for oxygen binding, cooperativity, BPG effects and what alterations in activity does single mutations have on structure and function. Myoglobin: 44 x 44 x 25 Å single subunit 153 amino acid residues 121 residues are in an a helix. Helices are named A, B, C, …F. The heme pocket is surrounded by E and F but not B, C, G, also H is near the heme. Amino acids are identified by the helix and position in the helix or by the absolute numbering of the residue. The Backbone structure of Myoglobin 4 The Heme complex in myoglobin Hemoglobin Spherical 64 x 55 x 50 Å two fold rotation of symmetry a and b subunits are similar and are placed on the vertices of a tetrahedron. There is no D helix in the a chain of hemoglobin. Extensive interactions between unlike subunits a2-b2 or a1-b1 interface has 35 residues while a1-b2 and a2-b1 have 19 residue contact. Oxygenation causes a considerable structural conformational change Quaternary structure of deoxy- and oxyhemoglobin T-state R-state Oxygenation rotates the a1b1 dimer in relation to a2b2 dimer about 15° The conformation of the deoxy state is called the T state The conformation of the oxy state is called the R state individual subunits have a t or r if in the deoxy or oxy state. What causes the differences in the conformation states? It is somehow associated with the binding of oxygen, but how? The positive cooperativity of O2 binding to Hb arises from the effect of the ligand-binding state of one heme on the ligand-binding affinity of another. The Fe iron is about 0.6 Å out of the heme plane in the deoxy state. When oxygen binds it pulls the iron back into the heme plane. Since the proximal His F8 is attached to the Fe this pulls the complete F helix like a lever on a fulcrum. Binding of the oxygen on one heme is more difficult but its binding causes a shift in the a1-b2 contacts and moves the distal His E7 and Val E11 out of the oxygen’s path to the Fe on the other subunit. This process increases the affinity of the heme toward oxygen. The a1-b2 contacts have two stable positions. These contacts, which are joined by different but equivalent sets of hydrogen bonds and act as a binary switch between the T and the R states The energy in the formation of the Fe-O2 bond formation drives the T R transition. Hemoglobins O2 -binding Cooperativity derives from the T R Conformational shift. •The Fe of any subunit cannot move into its heme plane without the reorientation of its proximal His so as to prevent this residue from bumping into the porphyrin ring. •The proximal His is so tightly packed by its surrounding groups that it can not reorient unless this movement is accompanied by the previously described translation of the F helix across the heme plane. •The F helix translation is only possible in concert with the quaternary shift that steps the a1C-b2FG contact one turn along the a1C helix. •The inflexibility of the a1-b1 and the a2-b2 interfaces requires that this shift simultaneously occur at both the a1-b2 and a2-b1 interfaces. No one subunit or dimer can change its conformation. The t state with reduced oxygen affinity will be changed to the r state without binding oxygen because the other subunits switch upon oxygen binding. Unbound r state has a much higher affinity for oxygen, and this is the rational for cooperativity a. Free energy changes with fractional saturation b. Sigmoidal binding curve as a composite of the R state binding and the T state binding. Hemoglobin function a2,b2 dimer which are structurally similar to myoglobin •Transports oxygen from lungs to tissues. •O2 diffusion alone is too poor for transport in larger animals. •Solubility of O2 is low in plasma i.e. 10-4 M. •But bound to hemoglobin, [O2] = 0.01 M or that of air •Two alternative O2 transporters are; •Hemocyanin, a Cu containing protein. •Hemoerythrin , a non-heme containing protein. Myoglobin facilitates rapidly respiring muscle tissue The rate of O2 diffusion from capillaries to tissue is slow because of the solubility of oxygen. Myoglobin increases the solubility of oxygen. Myoglobin facilitates oxygen diffusion. Oxygen storage is also a function because Myoglobin concentrations are 10-fold greater in whales and seals than in land mammals The Heme group Each subunit of hemoglobin or myoglobin contains a heme. •Binds one molecule of oxygen •Heterocyclic porphyrin derivative •Specifically protoporphyrin IX The iron must be in the Fe(II) form or reduced form. (ferrous oxidation) state. Loss of electrons oxidation LEO Gain of electrons reduction GER Leo the lion says GER The visible absorption spectra for hemoglobin The red color arises from the differences between the energy levels of the d orbitals around the Ferrous atom. There is an energy difference between them, which determines the size of the wavelength of the maximal absorbance band. Fe(II) = d6 electron configuration: Low spin state Binding of oxygen rearranges the electronic distribution and alters the d orbital energy. This causes a difference in the absorption spectra. Bluish for deoxy Hb Redish for Oxy Hb Measuring the absorption at 578 nM allows an easy method to determine the percent of Oxygen bound to hemoglobin. When Fe(II) goes to Fe(III), oxidized, it produces methemoglobin which is brown and coordinated with water in the sixth position. Dried blood and old meat have this brown color. Butchers use ascorbic acid to reduce methemoglobin to make the meat look fresh!! There is an enzyme methemoglobin reductase that converts methemoglobin to regular hemoglobin. O2 binding to myoglobin Written backwards we can Mb O2 MbO2 get the dissociation constant [Mb][O 2 ] Kd [MbO 2 ] Fractional Saturation solve for [MbO2] and plug in [MbO 2 ] [O 2 ] YO 2 [Mb] [MbO 2 ] Kd [O 2 ] How do you measure the concentration of oxygen? Use the partial pressure of O2 or O2 tension. = pO2 pO 2 YO 2 K d pO 2 pO 2 YO 2 P50 pO 2 P50 = the partial oxygen pressure when YO2 = 0.50 What is the shape of the curve if you plot YO2 vs. pO2 What does the value of P50 tell you about the O2 binding affinity? P50 value for myoglobin is 2.8 torr or 1 torr = 1 mm Hg = 0.133 kPa 760 torr = 1 atm of pressure Mb gives up little O2 over normal physiological range of oxygen concentrations in the tissue i.e. 100 torr in arterial blood 30 torr in venous blood YO2 = 0.97 to YO2 = 0.91 What is the P50 value for Hb? Should it be different than myoglobin? The Hill Equation E = enzyme, S = ligand, n= small number E nS ESn This is for binding of 1 or more ligands O2 is considered a ligand n [E][S] 1. K [ESn] n[ESn] 2. Ys n [E] [ESn] Fractional Saturation = bound/total As we did before, combine 1. + 2. = 3. n [E][S] K Ys n 1 [S] [E] K n 3. or [S] Ys n K [S] Look similar to Mb + O2 except for the n Continuing as before: K P50 n pO 2 n n P50 pO2 n 4. YO2 n = Hill Constant, a non integral parameter relating Degree of Cooperativity among interacting ligandbinding sites or subunits The bigger n the more cooperativity (positive value) If n = 1, non-cooperative n < 1, negative cooperativity Hill Plot Rearrange equation 4. Ys Log nLog[S] logK 1 - Ys y = mx + n = slope and x intercept of -b/m b Things to remember Hb subunits independently compete for O2 for the first oxygen molecule to bind When the YO2 is close to 1 i.e. 3 subunits are occupied by O2 , O2 binding to the last site is independent of the other sites However by extrapolating slopes: the 4th O2 binds to hemoglobin 100 fold greater than the first O2 A DDG of 11.4 kJ•mol -1 in the binding affinity for oxygen When one molecule binds, the rest bind and when one is released, the rest are released. Contrast Mb O2 binding to Hemoglobin YO2 = 0.95 at 100 torr but 0.55 at 30 torr a DYO2 of 0.40 Understand Fig 9-3 Hb gives up O2 easier than Mb and the binding is Cooperative!! Function of the globin Protoporphyrin binds oxygen to the sixth ligand of Fe(II) out of the plane of the heme. The fifth ligand is a Histidine, F8 on the side across the heme plane. His F8 binds to the proximal side and the oxygen binds to the distal side. The heme alone interacts with oxygen such that the Fe(II) becomes oxidized to Fe(III) and no longer binds oxygen. Fe O O Fe A heme dimer is formed which leads to the formation of Fe(III) By introducing steric hindrance on one side of the heme plane interaction can be prevented and oxygen binding can occur. The globin acts to: •a. Modulate oxygen binding affinity •b. Make reversible oxygen binding possible The globin surrounds the heme like a hamburger is surrounded by a bun. Only the propionic acid side chains are exposed to the solvent. Amino acid mutations in the heme pocket can cause autooxidation of hemoglobin to form methemoglobin. The Bohr Effect Higher pH i.e. lower [H+] promotes tighter binding of oxygen to hemoglobin and Lower pH i.e. higher [H+] permits the easier release of oxygen from hemoglobin Hb O 2 n H x O 2 Hb O 2 n 1 xH Where n = 0, 1, 2, 3 and x 0.6 A shift in the equilibrium will influence the amount of oxygen binding. Bohr protons Origin of the Bohr Effect The T R transition causes the changes in the pK’s of several groups. The N-terminal amino groups are responsible for 20 -30% of the Bohr effect. His146b accounts for about 40% of the Bohr effect salt bridged with Asp 94b. This interaction is lost in the R state. To help you understand look at the relation between pH and the P50 values for oxygen binding. As the pH increases the P50 value decreases, indicating the oxygen binding increases. The opposite effect occurs when the pH decreases. At 20 torr 10% more oxygen is released when the pH drops from 7.4 to 7.2!! CO2 H2O H HCO3 - As oxygen is consumed CO2 is released. Carbonic Anhydrase catalyzes this reaction in red blood cells. About 0.8 mol of CO2 is made for each O2 consumed. Without Carbonic Anhydrase bubbles of CO2 would form. The H+ generated from this reaction is taken up by the hemoglobin and causes it to release more oxygen. This proton uptake facilitates the transport of CO2 by stimulating bicarbonate formation. R-NH2 + CO2 R-NH-COO- + H+ Carbomates are formed with the interaction of CO2 with the Nterminal amino groups of proteins. About 5% of the CO2 binds to hemoglobin but this accounts for the 50% of the exchanged CO2 from the blood. As oxygen is bond in the lungs the CO2 comes off. Lecture 3/12/2003 Protein Function II Globins and Antibodies