* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Polypeptide and protein structure
Survey
Document related concepts
Vesicular monoamine transporter wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Ligand binding assay wikipedia , lookup
Proteolysis wikipedia , lookup
Point mutation wikipedia , lookup
Protein structure prediction wikipedia , lookup
Mineralized tissues wikipedia , lookup
Drug design wikipedia , lookup
Genetic code wikipedia , lookup
Metalloprotein wikipedia , lookup
Biochemistry wikipedia , lookup
Transcript
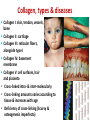
Structure-Function relationship Nafith Abu Tarboush DDS, MSc, PhD [email protected] www.facebook.com/natarboush The heme Myoglobin The first to be determined structurally A single polypeptide chain (153 a.a) A single heme in a hydrophobic pocket 8 -helices; no -sheets Most polar (exterior) Nonpolar (interior) Two His residues Fe(II) coordination Hemoglobin • A tetramer 22: -chains (141 a.a) & -chains (146 a.a) • 1 heme group in each (4O2) • Myoglobin (storage) vs. hemoglobin (transport): positive cooperativity & saturation percentage • lungs (100 torr), capillaries (20 torr) Conformational Changes Accompany Hb Function Oxygenated vs. deoxygenated (different crystal structures) Bohr effect: (tissues vs. lungs): lower pH → ↑H+→ protonation (ex. His146) → Asp94- His146 salt bridge → lower O2 affinity Oxy form is a stronger acid (less H+ affinity) than de-oxy (higher H+ affinity) CO2 (tissues vs. lungs) → H2CO3 → HCO-3+H+ → ↓ pH (less O2 affinity) O2 binding affinity, CO2 & H+ in myoglobin Conformational Changes Accompany Hb Function 2,3 BPG have an allosteric binding, lower affinity Hb F has a higher affinity for O2 due to: α2γ2 Less affinity to BPG (adult hemoglobin, βHis143-BPG salt bridge, fetal hemoglobin, the γ –Ser instead of His) The Collagen Triple Helix The most abundant protein in vertebrates Organized in water-insoluble fibers Have a great strength Consists of three polypeptide chains wrapped around each other in a ropelike twist, or triple helix Has a repeating sequence of the amino acids; X1—X2(Pro, ProOH)—Gly Hydroxy-lysine also occurs in collagen The triple helix (tropocollagen) is 300 nm long and 1.5 nm in diameter Held together by H-bonding Each strand have ≈800 amino acids (300KDa) Collagen, types & diseases Collagen I: skin, tendon, vessels, • • • bone Collagen II: cartilage Collagen III: reticular fibers, alongside type I Collagen IV: basement membrane Collagen V: cell surfaces, hair and placenta Cross-linked intra- & inter-molecularly Cross-linking amounts varies according to tissue & increases with age Deficiency of cross-linking (Scurvy & osteogenesis imperfecta) Keratin • Principal component of epidermis and related appendages (hair, horn, nails, & feathers) • α(mammals) or β(birds & reptiles) • Mammals: ≈30 types, tissue-specific • Structure: α-helix, coiled coil Elastin • Rich in hydrophobic amino acids (ex. Gly, Val & Pro); mobile hydrophobic regions bonded by crosslinks between Lys • Elastic fibers in arteries are composed mainly of elastin (≈70%) • Tropoelastin → Elastin (Lysyl oxidase) Elastin & hydroxylysine • Collagen contain lysine that can be hydroxylated by lysyl-hydroxylase to form hydroxyl-lysine or by lysyl-oxidase to form Allysine • Cross-linking of elastin occurs through the enzyme lysyl-oxidase producing the Allysine, the pathway for oxidation through lysylhydroxylase does not occur in elastin