* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Safety of COX
Survey
Document related concepts
Metalloprotease inhibitor wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Drug-eluting stent wikipedia , lookup
Neuropharmacology wikipedia , lookup
Clinical trial wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Theralizumab wikipedia , lookup
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
Transcript
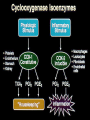
The Safety of COX-2 Inhibitors John J. Cush, MD Presbyterian Hospital of Dallas GI Outcomes Trials: Design VIGOR (n=8076) CLASS (n=7982) Drug Rofecoxib 50 mg QD (2x max chronic dose) Celecoxib 400 mg BID (2x max chronic dose) Patients RA OA (72 %), RA (28 %) Comparator Naproxen 500 mg BID Ibuprofen 800 mg TID Diclofenac 75 mg BID Low dose ASA No Yes (21 %) Duration Median 9 months Maximum 13 months 6 months reported Median 9 months Maximum 13 months Bombardier et al. N Engl J Med. 2000;343:1520-1528 Silverstein et al. JAMA. 2000; 284:1247- NSAID Impact/Cost 13 million chronic users (70 million Rx/yr) Dyspepsia 20% > Gastric ulcers 10% Serious GI complications 1.3 % RA pts 0.73% OA pts Hospitalization (UGI bleed) 103,000/year 5-10% mortality (est 16,500 deaths/year) NSAID deaths 15th most common cause in USA Cost > $2 billion/year NSAID Concerns 13 million chronic users (U.S.) Dyspepsia in 10-20% Serious GI complications in 1.3% Mortality for 7 Selected Disorders (1997) Fatalities in 5-10% of these Direct cost/year > $2 billion ~ 15th most common cause of death (U.S.) Wolfe, et al: NEJM 1999 Mechanism of Action of NSAIDs CO2H Arachidonic acid COX-1 “Constitutive” COX-2 “Inducible” Non-specific NSAIDs COX-2 NSAIDs GI Mucosa Platelet Prostaglandins Prostaglandins Thromboxane GI mucosal Protection Hemostasis Bakhle et al. Med Inflamm. 1996;5:305-323. Vane et al. Inflamm Res. 1995;44:1-10. Mediate pain, inflammation, and fever GI Outcomes Trials: Design VIGOR (n=8076) CLASS (n=7982) Drug Rofecoxib 50 mg QD (2x max chronic dose) Celecoxib 400 mg BID (2x max chronic dose) Patients RA OA (72 %), RA (28 %) Comparator Naproxen 500 mg BID Ibuprofen 800 mg TID Diclofenac 75 mg BID Low dose ASA No Yes (21 %) Duration Median 9 months Maximum 13 months 6 months reported Median 9 months Maximum 13 months Bombardier et al. N Engl J Med. 2000;343:1520-1528 Silverstein et al. JAMA. 2000; 284:1247- CLASS Trial: Upper GI Complications Alone and With Symptomatic Ulcers = celecoxib = NSAIDs (ibuprofen + diclofenac) Patients Not Taking Aspirin Patients Taking Aspirin p = 0.02 5 4 2 1 49 / 1384 p = 0.09 3 Annualized Incidence % All Patients 6 11 / 1441 20 / 1384 30 / 1441 0 6 p = 0.02 5 4 3 2 1 p = 0.04 14 / 1101 5 / 1143 32 / 1101 16 / 1143 0 p = 0.49 17 / 283 6 14/ 298 5 4 p = 0.92 3 2 6 / 298 6 / 283 1 0 Silverstein et al. JAMA 2000; 284:1247-1255 Ulcer ComplicationsSymptomatic Ulcers and Ulcer Complications Events Leading up to FDA Mtg Results of VIGOR and CLASS 9/30/04 Merck voluntary w/d of Vioxx (based on APPOVE trial) Reanalyses of all COX2 data 12/9/04 FDA warning of CV events in Bextra CABG trial 12/17/04 NCI stop APC trial (concerns over celecoxib data) 12/20/04 NIH stops ADAPT trial (concerns over naproxen) FDA: COX-2 Safety February 16-18, 2005 Arthritis Advisory Committee Drug Safety and Risk Management Other speakers, SGEs 8 rheums, 19 physicians, 8 statisticians, 1 ethicist, patient and industry representatives ISSUES: 1. Does the agent pose a risk for CV events?; 2. Does the risk versus benefit profile of the drug support its marketing in the U.S.? 3. If continued marketing is supported, what actions are recommended to ensure its safe use? FDA COX-2 Hearing Limitations of available data Most trials of short duration, using low dose Most trials done with efficacy endpoints Few designed with CVS safety endpoint Most trials were active comparator and NOT placebo controlled trials Observational studies (presented) are for generating signals and hypotheses Safety signals drawn from other indications are valid, but not conclusive unless repeated or of sufficiently great magnitude Table 1A. Placebo-controlled trials: Prospective CV Endpoint* Trial N Populatio n COX-2 dose/d (Rx duration) APPROV Polyp Rofecoxib 25 2586 E** prevention mg (3 yr) CABGII** CABG-I (035)** Control Results Placebo Cardiac evnt 31 v 12; HR 2.8 (CI, 1.4-5.4) High risk 1671 CABG Valdecoxib 40 mg Parecoxib 40 mg (10 d) CV/thromboembolic Placebo 11 v 3; RR 3.7 (CI, 1-13.5) 462 Parecoxib IV 80mg Valdecoxib 80 mg (10 days) No increase in MI; Increased CVA in Placebo parecoxib/ valdecoxib (2.9% v 0.7%) CABG Table 1B. Placebo-controlled trials: Sporadic, Observed CV Adverse Events Trial APC N 203 5 Alzheimer 425 s-001 Population COX-2, dose/day (Rx duration) Control Results Polyp prevention Celecoxib 400 mg Celecoxib 800 mg (>2.8 yrs) Placebo HR 400mg RR 3.0 (0.3-28.6) 800 mg RR 6.1 (0.750.3) Alzheimer’s prevention Celecoxib 400 mg (1 yr) Placebo MI 2 v 0; CV deaths 2.4 v 1.4% Pre-SAP 156 1 Polyp prevention Celecoxib 400 mg Placebo No CV increase ADAPT+ 246 3 Alzheimer’s prevention Celecoxib 400 mg (~2 yrs) Placebo Naproxen No CV increase Alzheimer 209 /MCI 1 Alzheimer’s prevention Rofecoxib 25 mg (1 yr) Placebo No CV increase Rofecoxib (3 yr) Placebo RR 3.14 (1-9.75) VICTOR$ 197 Polyp 6 PY prevention Table 2A. Naproxen active comparator trials: Observed Adverse events Trial N Populati COX-2,dose Compared Results VIGOR 8076 RA Rofecoxib 50mg/10 mo Naproxen MI: 20 v 4 @ 8mos RR 2.38 (1.39-4.0) Etoricoxib 3457 Metaan alyses Etoricoxib Naproxen RR 1.70 (0.9-3.18) TARGET (0117)** 9471 OA Lumiracoxib 400 mg 1 yr) Naproxen HR 1.77 (0.82-3.84) Table 2B. NSAID active comparator trials: Observed Adverse events CLASS** 5968 Success-1 13194 Celebrex800 Diclofenac mg (9-5mos) Ibuprofen No CV increase OA Celebrex 200, 400 mg Naproxen Diclofenac No CV increase OA, RA EDGE** 7111 OA/GI tolerab Etoricoxib (9-16 mos) Diclofenac No CV increase Small ↑ HTN TARGET (2332)** 8773 OA Lumiracoxib 400 mg/1 yr Ibuprofen No CV increase Serious CV/T1 Events Different Endpoints (VIGOR) Vioxx 50 mg 64 Naproxen 45 35 32 19 Investigator CV/ T 1 Adjudicated 2 18 APTC 3 Cardiovascular Thrombotic. 2Confirmed by CV adjudication committee. 3 Antiplatelet Trialist Collaboration composite endpoint: CV & unknown cause of death, non-fatal myocardial infarction and non-fatal stroke. Vioxx - APPROVe APTC Events Time to Event Plot Rofecoxib 25 Placebo Vioxx – APPROVe: Effect of ASA on APTC * Vioxx 25 Placebo RR, p-value (95% CI) 1287 1299 33/3053 16/3322 Rate/100 PYR 1.08 0.48 Non-ASA user 1074 1095 28/2564 12/2817 1.09 0.43 213 204 n/PYR 5/489 4/505 1.29 Rate/100 PYR 1.02 0.79 (0.28,6.50) All patients n/PYR n/PYR Rate/100 PYR ASA user * Based on October 2004, submission (no final dataset). 2.25 P=0.008 2.57 (1.31,5.06) CV Signal in Vioxx Databases AAC 1998 NDA Versus Plac+NSAIDs 2000 VIGOR Napr Labeling changes 2001-2002 102 RAe SURs/AD ----Epi and re-analyses----Napr Napr Placebo N Y t t N CV Death N N t t t NF MI NF Stroke N Y t t N N N N N N APTC N= no signal. Y= Clear signal. t = Trend. AAC: February 8, 2001 FDA Arthritis Advisory Committee meeting. RAe: Rheumatoid Arthritis efficacy supplement. SURs/AD: Safety Update Reports including Alzheimer’s disease studies. Epi= epidemiologic studies. NF= non-fatal. CLASS - APTC-like Events Event Celebrex (n=3987) Diclofenac (n=1996) Ibuprofen (n=1985) CV death 11 5 5 MI 19 4 9 Stroke 4 6 6 TOTAL 34 (0.9%) 15 (0.8%) 20 (1.0%) FDA COX-2 Hearing Vote: Retain on the market Celecoxib 32-0 Valdecoxib 17-13 (2 abstentions) Rofecoxib 17-15 Unanimously in favor of: ? “Black box” warning for CV risk & COX-2 drugs Education measures for pts and physicians Restrictions on direct-to-consumer advertising “Warning” added to Current NSAIDs Compassionate use liquid rofecoxib for kids Summary COX-2 inhibitors equipotent to NSAIDs GI Toxicity lower with COX-2, but negated by low dose ASA 81 mg/d Would this be off-set by use of PPI CV risk modestly higher w/ COX-2 inhibitor Same for NSAIDs? Not affected by use of low dose ASA FDA: COX-2 Safety Risk/benefit of COX-2 inhibitors favors continued use in U.S Patients should be counseled about cardiovascular risks. While all NSAIDs may impose similar cardiovascular risks, some agents (naproxen 500 mg bid, celecoxib 200 mg qd) appear to be safer than others (e.g., rofecoxib, valdecoxib, diclofenac, and ibuprofen). Cardiovascular risks of the COX-2 inhibitors may be greater with higher doses, longer durations of therapy, and when used in high risk individuals. The use of low dose aspirin does not consistently abrogate the potential CV risk of a COX-2 inhibitor. Patients who require the cardioprotective effects of aspirin may not be ideal candidates for COX-2 inhibitor or NSAID therapy. The use of COX-2 inhibitors (and other NSAIDs thought to increase CV risk) should be avoided in high risk individuals Bextra FDA Action: 4/7/05 We have concluded that the overall risk versus benefit profile for this product is unfavorable and we have requested Pfizer to voluntarily withdraw the product from the market. They have agreed to suspend sales and marketing in the U.S., pending further discussions with the Agency. To resume marketing of the drug, the sponsor would have to demonstrate that Bextra has a clear benefit over existing therapies or a lower risk compared to other COX-2 selective inhibitors. Celebrex We have concluded that Celebrex should remain available as a prescription drug, but with changes to the labeling, a MedGuide, and commitments from Pfizer for additional studies to better define the cardiovascular effects of the drug. Vioxx A proposal by Merck to reintroduce Vioxx to the market would require a supplemental new drug application. Such an application would be reviewed with consideration of the risk to benefit balance of the proposed indications and populations for use, warnings in the label, and all relevant data. We expect that the proposal would also be reviewed at a public Advisory Committee meeting. Prescription Non-Selective NSAIDs- Based upon the available data, we have concluded that an increased risk of CV events may be a class effect for NSAIDs. Therefore, at this time, changes to the prescribing information for all of these drugs are warranted, until the risk profile of the individual agents can be better assessed. Non-prescription Non-Selective NSAIDs- The labeling for low dose, non-prescription products that contain ibuprofen, naproxen and ketoprofen will be revised to include warnings about potential CV and GI risks, advisories recommending certain patients seek physician input before use and stronger reminders to follow the instructions of the label concerning dose and duration of treatment A New Standard for Drug Development Any new NSAID, must be clinically better than current alternatives Any new NSAID, must be safer Usual phase IV trials must be done before approval, not after. NSAIDs Available by Prescription NSAIDs SALICYLATES Diclofenac (Voltaren) Diclofenac/Misoprostol (Arthrotec)b Fenoprofen (Nalfon) Flurbiprofen (Ansaid) Ibuprofen (Motrin)a Indomethacin (Indocin) Ketoprofen (Orudis)a Meclofenamate Mefenamic acid (Ponstel) Nabumetone (Relafen) Naproxen (Naprosyn, Anaprox)a Oxaprozin (Daypro) Piroxicam (Feldene) Sulindac (Clinoril) COX-2 INHIBITORS Aspirina (Zorprin, Easprin) Diflunisal (Dolobid) Salsalate (Disalcid, Salflex) Choline salicylate (Trilisate) Magnesium salicylate (Magan) Celecoxib (Celebrex) Valdecoxib (Bextra) In Development Etoricoxib Parecoxibc Lumiracoxib Previously Available Rofecoxib (Vioxx) Tolmetin (Tolectin) a Also b c available as over-the-counter preparations in the U.S. Combination tablet of NSAID/synthetic prostaglandin E1 Parenterally administered 2004 Physician’s Desk Reference In Vitro Selectivity: COX-2/COX-1 Ratio lumiracoxib etoricoxib rofecoxib valdecoxib > 50-fold COX-2 selective etodolac nimesulide diclofenac celecoxib meloxicam 5- 50-fold COX-2 selective fenoprofen < 5-fold COX-2 ibuprofenselective tolmetin naproxen aspirin indomethacin ketoprofen flurbiprofen ketorolac Warner et al. FASEB J. 2004:18:790-804 -3 -2 -1 Increasingly COX-2 Selective 0 1 2 Increasingly COX-1 Selective 3 Range of COX Selectivity for COX-1 and COX-2 (log10 IC50 COX-2/COX-1) Figure 1. Pooled and study specific relative risks and 95% confidence intervals for the risk of myocardial infarction associated with use of NSAIDs as a class, naproxen and ibuprofen. RR OF MYOCARDIAL INFARCTION Garcia-Rodriguez I Ray I Garcia Rodriguez 2004 Solomon Watson Schlienger Ray II Mamdani Kurth Kimme l Garcia-Rodriguez II Pooled NSAIDs NSAIDs RR=1.04 [1.00-1.08 Garcia-Rodriguez I Ray I Solomon Watson Schlienger Ray II Mamdani Kimmel NAPROXEN RR=0.88 [0.8-0.95] Garcia-Rodriguez II Pooled Naproxen Garcia-Rodriguez I Ray I Solomon Watson Ray II Kimmel IBUPROFEN Garcia-Rodriguez II RR = 1.03 [.96-1.1] Pooled Ibuprofen 0 1 2 ? : Study specific, adjusted RRs; ? Pooled RR; — 95% C NAPROXEN : HALF AS GOOD AS ASPIRIN? Long-term NSAID Use May Increase Risks of Cardiovascular Death-Norwegian Study, 2005 Population-based, nested case-control study of 454 Scandinavian patients diagnosed with oral cancer between 1975 and 2003, and 454 gender- and agematched controls. (Sudbo J, et al. 2005 - Manuscript submitted) CV deaths 2.06 Any NSAID 42 1.16 2 Aspirin 2.86 Ibuprofen 1.70 Naproxen 7 2.26 Indomethacin 10 1.84 Piroxicam 7 1.90 Ketoprofen 0 12 1 4 2 3 4 5 6 Hazard Ratio for CV Death in Long-term NSAID Users Compared to Non-users (with 95% Confidence Intervals) On Safety… “The desire for safety stands against every great and noble enterprise” - Tacitus “ I think we too often make choices based on the safety of cynicism, and what we’re lead to is a life not fully lived” - Ken Burns