* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download ANTIGLAUCOMA MEDICATIONS
Survey
Document related concepts
Psychedelic therapy wikipedia , lookup
Orphan drug wikipedia , lookup
Compounding wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Discovery and development of beta-blockers wikipedia , lookup
Theralizumab wikipedia , lookup
Drug design wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Neuropharmacology wikipedia , lookup
Psychopharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Drug discovery wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Transcript
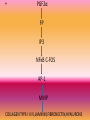
ANTIGLAUCOMA MEDICATIONSPART I DR PRATIBHA THILAK PHARMACOKINETICS OF TOPICAL DRUGS • a) drug kinetics in the conjunctival cul-de-sac, • (b) corneal and transconjunctival-scleral penetration, and • (c) the distribution and rate of drug elimination within the eye DRUG KINETICS IN THE CONJUNCTIVAL CUL-DE-SAC • first mixes with the tears in the cul-de-sac • depends on blink movements for mixing with instilled drugs • 7 to 9µl of tears and has a maximum capacity of about 30 µl • 25.1 to 56.4 µl with an average of 39 µl • enters the lacrimal drainage system as a result of the pumping action created by blink movements • DRUG LOSS FROM TEARS • Decreases bioavailability • Increases systemic toxicity • nasolacrimal occlusion CORNEAL AND TRANSCONJUNCTIVALSCLERAL PENETRATION • cornea as lipid-water-lipid sandwich • selective barrier • differential solubility concept • drugs tend to concentrate in various layers of the cornea • depot and a limiting factor for transfer of the drug to the aqueous humor • enzymes that are important in activating some glaucoma drugs to their active form, such as dipivefrin and bimatoprost INTRAOCULAR FACTORS INFLUENCING DRUG CONCENTRATIONS • factors affecting the bioavailability of the drug • intraocular circulation, local tissue binding, and local tissue metabolism • eliminated by diffusion into the vascular system or escapes with the aqueous via the outflow system • bound to various ocular tissues FORMULATIONS OF TOPICAL DRUGS • vehicle, pH, concentration, and additives of the formulation VEHICLES • influencing the rate of drug loss in the tears, the precorneal tear film saturation, and the length of time that the drug remains in contact with the cornea • methylcellulose and polyvinyl alcohol • increasing tear viscosity, providing solution homogeneity (uniform suspension of drug particles in solution), and reducing surface tension • Ointments significantly increase drug bioavailability by • reducing loss in the tears, • inhibiting dilution by the tears, • providing a higher effective concentration of the drug, and • increasing tissue contact time • polysaccharide gum formulations • Liposomes • small, homogenous unilamellar structures composed of phospholipids and water • Temperature-sensitive liposomes, which release substances in the ocular vasculature when exposed to heat produced by an argon laser or microwave solid materials • release a drug at a sustained rate from a location on the cornea or in the conjunctival cul-de-sac • • • • water-soluble polymer matrices insoluble material presoaked hydrophilic contact lenses collagen corneal shields pH, MOLECULAR WEIGHT, AND DROP SIZE • A greater degree of corneal penetration occurs when a higher concentration of nonionized (lipid soluble) drug exists in the instilled drop • Weak bases absorbed through the cornea at a higher pH, while weak acids are absorbed better at a lower pH • molecular weight greater than 500 g/mole have poor corneal absorption • CONCENTRATION • enhanced by increasing the concentration of a drug up to a point • decreases with increasing concentrations, and the greater amount of drug lost in the lacrimal drainage system increases the potential for systemic side effects ADDITIVES • benzalkonium chloride and benzododecinium bromide • providing bacteriostatic activity, • decreases the surface tension of nonpolar drugs, allowing them to mix more readily with the precorneal tear film, leading to enhanced corneal absorption PHARMACOGENETICS • Compliance • biological mechanisms related to aqueous humor dynamics ocular and systemic conditions • environmental factors and genetics PROSTAGLANDINS AND HYPOTENSIVE LIPIDS • Eicosanoids • Inactivated in lung and liver Endogenous –PGD2,PGE2,PGF2α,PGI2,TXA2 MECHANISM OF ACTION • • • • PROSTANOID RECEPTORS EP,FP,IP,TP GPCR FP-type A,type B • RELAXATION OF CILIARY MUSCLE • REMODELLING OF EXTRACELLULAR MATRIX • SHORT ONSET , LONG LASTING RELAXATION, NARROWING OF MUSCLE FIBRE BUNDLES • PGF2α FP IP3 NFκB C-FOS AP-1 MMP COLLAGEN TYPE-I II IV,LAMININ,FIBRONECTIN,HYALURONS LATANOPROST • 0.005%.ONCE DAILY • LOWERS IOP AT NIGHT AND DAY AS WELL • UNIFORM ROUND THE CLOCK IOP REDUCTION WHEN USED ALONE OR IN COMBINATION • SAFE BUT LESS EFFECTIVE IN CHILDREN • BETTER RESPONSE IN OLDER CHILDREN AND JUVENILE ONSET OAG EFFICACY STUDIES • As effective as timolol in chronic ACG • Effective in lowering IOP when IOP elevation persists after peripheral iridectomy UNOPROSTONE • 0.15%, 0.12% • Not as effective as timolol and latanoprost • Increase activity of inhibitors MMP TRAVOPROST • 0.004% • MORE EFFECTIVE THAN TIMOLOL IN SOME STUDIES • AS EFFECTIVE AS LATANOPROST • Lowers IOP in chronic ACG BIMATOPROST • 0.03% • AMIDE ETHYL GROUP AT C1 POSITION • PROSTAMIDE • HYDROLYZED BY CORNEA TO A LESSER EXTENT • Fp receptor agonist + trabecular outflow+ alternative signalling pathway EFFICACY • Greater IOP lowering efficacy than timolol • Similar IOP lowering effect as latanoprost • Lower IOP in chronic ACG ADMINISTRATION • ONE DROP DAILY • UNOPROSTONE-TWO DROPS DAILY • SHORT T1/2 • IV-9.2 MINS,TOPICAL-2.3 MINS • THERMAL AND ULTRAVIOLET INSTABILITY • STORED UNDER REFRIGERATION DRUG INTERACTION • LATANOPROST 0.005% + TIMOLOL 0.5% • TRAVOPROST 0.004% + TIMOLOL 0.5% • ADDITIVE EFFECT WITH MIOTICS SIDE EFFECTS • Greater conjunctival hyperaemia than timolol treated eyes • Latanoprost increased number of fibroblasts,goblet cells • h/o herpes simplex keratitis • Cautious use of this class of drugs is advised in eyes with risk factors for cystoid macular edema • increased pigmentation in the periocular skin and iris, and alterations in the eyelid cilia • melanin content is increased in iris stromal melanocytes without proliferation of cells • mechanism of increased skin pigmentation appears to be related to enhancing tyrosinase activity , and altering the chemistry of the melanin • alterations in eyelid cilia include hypertrichosis and increased pigmentation • stimulating the growth phase of the hair cycle in the dermal papilla • Approval of bimatoprost 0.03% for treatment of hypotrichosis of eyelashes • allergic contact dermatitis to latanoprost ,iris cyst associated with latanoprost and herpes simplex dermatitis • No significant systemic reactions Little effect on cardiovascular and respiratory systems Β ADRENERGIC RECEPTOR ANTAGONIST MECHANISM OF ACTION • Reducing aqueous humor production • β2 subtype found on ciliary processes and TM • Inhibition of c-AMP • Outflow facility not influenced • Does not influence blood aqueous barrier TIMOLOL • Timolol maleate and hemihydrate • 0.25 %,0.5% Non selective β1,β2 antagonist Iop lowering effect in treated and fellow untreated eye EFFICACY • Epinephrine-greater • Pilocarpine-equivalent or slightly greater BETOXALOL • Cardioselective • β1 adrenergic antagonist • 0.25% • Efficacy less than timolol LEVOBUNALOL • Non selective β1,β2 antagonist • 0.5% ONCE DAILY • SIMILAR IN EFFICACY TO TIMOLOL,METIPRANOLOL • GREATER EFFICACY THAN BETAXOLOL CARTEOLOL Non selective β antagonist intrinsic sympathomimetic activity 1%, 2% May decrease cardiovascular risks a/w cholesterol abnormaliies Less ocular irritation than timolo METIPRANOLOL • Non selective β antagonist • 0.3% • COMPARABLE WITH TIMOLOL AND LEVOBUNOLOL IN EFFICACY AND SAFETY ATENOLOL • selective β1 antagonist • 2% METOPROLOL • Cardioselective • β1 adrenergic antagonist • 1-5% • EQUIVALENT EFFICACY TO TIMOLOL AND PILOCARPINE PINDOLOL • Potent βadrenergic antagonist with intrinsic sympathomimetic activity • 0.5-1% NADOLOL • NON SELECTIVE β BLOCKER • 1-2% • DIACETYL NADOLOL • LOWER INCIDENCE OF TOLERANCE BEFUNOLOL • 0.25-0.5% ADMINISTRATION • Individuals with darker irides require higher concentrations of timolol • Non specific binding of drug to pigment FREQUENCY • IOP lowering effect peaks at 2 hrs and lasts for 24 hrs • Twice daily • On discontinuation-no increase significantly till 4th day • Effect seen till 14 days • Melanotic tissur and slow release LONG TERM EFFICACY • the pressure responsiveness to timolol will decrease with continued administration. • Occurs in two phases, which Boger called short-term escape and long-term drift. Short term escape • dramatic reduction in IOP with the initiation of timolol therapy • rises during the next few days and plateaus at a maintenance level • • EXPLANATION-number of β-receptors in ocular tissues increases during the first few days of timolol therapy • • In any case, it is good clinical practice to wait approximately 1 month after initiating timolol to determine the efficacy of therapy LONG TERM DRIFT • slow decline in pressure response to timolol, usually beginning 3 months to 1 year after starting treatment • some regain responsiveness to timolol after a washout period • a treatment strategy of pulsatile therapy, in which 0.5% timolol is given for 6 months and then alternates with dipivefrin for 2 months, was studied and shown to minimize long-term drift compared with continuous use of timolol MULTIPLE AGENT FORMULATIONS • BRIMONIDINE 0.2% + TIMOLOL 0.5% • DORZOLAMIDE 2% + TIMOLOL 0.5% • PILOCARPINE 2-4 % + TIMOLOL 0.5% SIDE EFFECTS • does not affect the pupillary size or accommodation. • The tear film may be altered in patients who have low baseline tear flow • Chronic therapy with timolol has been shown to affect the mucus layer of the tear film • Burning and conjunctival hyperaemia a/w superficial punctate keratopathy and corneal anesthesia • serious side effect -ocular cicatricial pemphigoid • chronic inflammation from the irritating effects of the antiglaucoma medications and/or preservatives The most troublesome ocular reaction that has been reported with metipranolol is a granulomatous anterior uveitis, characterized by mutton-fat keratitic precipitates, flare and cells, and IOP elevation SYSTEMIC TOXICITY • MORE THAN OCULAR REACTIONS • SYSTEMIC ABSORPTION-8 MINS • the role of punctal occlusion CARDIOVASCULAR EFFECT • • • • • BRADYCARDIA WEAKENS MYOCARDIAL CONTRACTILITY ARRTHYTHMIAS SYNCOPE MORE WHEN CONCURRENTLY USED WITH OTHER DRUGS SUCH AS QUINIDINE AND CALCIUM CHANNEL BLOCKERS RESPIRATORY EFFECTS • CONTRACTION OF BRONCHIAL SMOOTH USCLE • BRONCHOSPASM AIRWAY OBSTRUCTION • ESP IN ASTHAMATICS • more common in young children, and caution must be taken by nursing mothers, because high levels of timolol were found in the milk of a mother receiving topical timolol • CENTRAL NERVOUS SYSTEM • depression, anxiety, confusion, dysarthria, hallucinations, lightheadedness, drowsiness, weakness, fatigue, tranquilization, dissociative behavior, disorientation, and emotional lability CHOLESTEROL LEVELS • 0.5% topical timolol twice daily for 2 months, without nasolacrimal occlusion, has been shown to decrease plasma HDL cholesterol levels, which increases the risk of coronary artery disease SYSTEMIC REACTIONS • gastrointestinal distress (nausea, diarrhea, and cramping) • • dermatologic disorders (maculopapular rash, alopecia, and hives) and sexual impotence • • exacerbation of myasthenia gravis • • altered response to hypoglycemic episodes in diabetic patients, which may mask the awareness of the attack α ADRENERGIC RECEPTOR ANTAGONISTS • THYMOXAMINE • MIOSIS BY INHIBITION OF DILATOR MUSCLE without influencing the ciliary muscle-induced facility of aqueous outflow • does not cause shallowing of the anterior chamber or ciliary spasm, it provides safe, rapid reversal of the effects of an adrenergic mydriatic drug. • 0.1% to reverse mydriasis from phenylephrine • role in management of angle-closure glaucoma • miosis despite pressure-induced ischemia of the iris sphincter and not increasing the posterior vector force of the iris, which might aggravate the pupillary block • thymoxamine produces miosis without affecting the ciliary muscle-controlled facility of outflow, it can break an angle-closure attack • narrowing of the palpebral fissure in many patients with eyelid retraction • especially cases secondary to thyroid disease, • value in the diagnosis of thyroid eye disease and possibly in the medical treatment of eyelid retraction (0.5%) DAPIPRAZOLE • reversal of mydriasis after an ocular examination • BUNAZOSIN • lower IOP in normal subjects in single doses of 0.025% to 0.2% and was effective for 1 week in a concentration of 0.1% • increased uveoscleral outflow • cause miosis, ptosis, and conjunctival hyperemia PRAZOSIN • topical administration of 0.001% to 0.1% prazosin causes a dose-related lowering of IOP by reducing aqueous humor formation CORYNANTHINE • Selective α1-adrenergic antagonist • increase in uveoscleral outflow LABETALOL • combined α1 and α2-adrenergic blocking agent • poor ocular hypotensive effect in human eyes THANK YOU !