* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Research
Vaccination wikipedia , lookup
Childhood immunizations in the United States wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Sociality and disease transmission wikipedia , lookup
Infection control wikipedia , lookup
Inflammation wikipedia , lookup
Kawasaki disease wikipedia , lookup
Schistosoma mansoni wikipedia , lookup
Pathophysiology of multiple sclerosis wikipedia , lookup
Management of multiple sclerosis wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Periodontal disease wikipedia , lookup
Behçet's disease wikipedia , lookup
Onchocerciasis wikipedia , lookup
Schistosomiasis wikipedia , lookup
Sjögren syndrome wikipedia , lookup
Globalization and disease wikipedia , lookup
Autoimmunity wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Germ theory of disease wikipedia , lookup
Rheumatoid arthritis wikipedia , lookup
Neuromyelitis optica wikipedia , lookup
Ankylosing spondylitis wikipedia , lookup
Crohn's disease wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Multiple sclerosis research wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
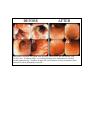
Ovamed GmbH TSO A NEW APPROACH TO THERAPY OF ULCERATIVE COLITIS AND CROHN'S DISEASE BEFORE AFTER Endoscopic evaluation of a patient with ulcerative colitis before and after therapy with Trichuris suis. Treatment with T. suis afforded healing of the inflammation as seen by flexible sigmoidoscopy. Numbers in upper left corner indicate location (centimeters from anal orifice) where photograph was made. Inflammatory Bowel Disease There are several diseases that fall within the grouping of inflammatory bowel disease (IBD). The two main divisions are Crohn’s disease and ulcerative colitis. Ulcerative colitis is further divided into ulcerative pancolitis (whole colon involved), left-sided ulcerative colitis (inflammation restricted to rectum through descending colon), and ulcerative proctitis (inflammation restricted to rectum). The term “indeterminate colitis” is used if a patient has an illness that could be either ulcerative colitis or Crohn’s because it shares features of both. The cause of IBD is not known. Some of the differences between Crohn’s disease and ulcerative colitis are listed in Table 1. Table 1: Characteristics of Crohn’s disease and ulcerative colitis Crohn’s disease Ulcerative colitis Inflammation can involve any part of GI tract. Normal areas can separate inflamed areas. Inflammation is transmural. Perianal inflammation is common. Strictures and fistulae occur. Inflammation recurs after resection. Inflammation limited to colon and rectum. Inflammation is contiguous from rectum. Inflammation only involves mucosa. Perianal inflammation is absent. Strictures and fistulae are absent. Inflammation does not recur after colectomy. Crohn’s disease can involve any part of the GI tract but ulcerative colitis is limited to the colon. Crohn’s disease can have normal areas of bowel separating inflamed areas. The inflammation in Crohn’s disease is “transmural” involving the mucosa, muscle layers, and serosa of the bowel. The inflammation is usually limited to the mucosa in ulcerative colitis. Because Crohn’s disease inflammation is transmural, strictures (narrowing of the bowel lumen due to scar) can form and cause obstruction. Also, this transmural inflammation can lead to fistulae (abnormal channels between organs). Fistulae can cause bowel contents to leak from openings to the skin (“enterocutaneous fistulae”), bladder (“enterovesicular fistulae”), vagina (“enterovaginal fistulae”), or luminal contents can escape into the abdominal cavity and cause abscess. An important difference between ulcerative colitis and Crohn’s disease is that Crohn’s disease recurs after surgery. Therefore, Crohn’s disease is not curable by surgery. Removing the colon can “cure” ulcerative colitis, but this is a very severe treatment option. Pathogenesis of IBD IBD results from an inappropriate inflammatory response to substances present in the intestinal lumen. The chronic intestinal inflammation may be triggered by a transient infection. However, the actual trigger is not identified in most patients. Current research efforts focus on determining what causes the inappropriate inflammation to persist. Basic and clinical research suggest that IBD results from a poorly regulated immune system. Support for this concept includes mice with various immune defects that develop colitis. In most of these mouse models, intestinal inflammation is associated with increased release of mucosal inflammatory cytokines like IL12, TNFα and IFNγ. Studies in patients show a similar increase in intestinal inflammatory cytokines (1-3). Genetics of IBD Genetic predisposition may play a role in IBD. For example, some patients with Crohn’s disease have mutations in a gene called Card15 or Nod2. Card15 is an intracellular protein expressed by monocytes and intestinal paneth cells (4) that recognizes bacterial muramyl dipeptide (5). It is unknown how Card15 mutation influences intestinal inflammation. Most (>80%) patients with Crohn’s disease do not have Card15 mutation and most people with Card15 mutations do not develop IBD. Mutations of Card15 do not associate with ulcerative colitis. It is likely that multiple gene interactions associated with environmental factors lead to IBD. Epidemiology of IBD There are many patients with IBD. The percentage of the population with IBD in northern Europe and the United Kingdom is rising and was recently estimated as 0.5% (6). IBD is a disease that became prevalent in the twentieth century in developed countries. There has been a dramatic rise in the incidence (onset of new cases) Crohn’s disease over the last century (Table 2). The incidence of IBD still seems to be increasing in many parts of the world (7;8). Improved diagnosis does not explain the dramatic increase in IBD and other autoimmune diseases (7;9). Table 2. New cases Crohn’s disease diagnosed yearly. New cases each year per 100,000 people Region Cardiff, Wales Minnesota, USA Derby, England N. Isles, Scotland Central Sweden Copenhagen, Denmark Beer Sheva, Israel 1930's 1940's 1950's 1960's 1970's 1980's 0.25 0.7 0.8 1.5 2.5 0.9 1.7 1.5 3.2 4.5 2.0 4.0 3.3 0.7 0.6 4.9 6.8 3.7 6.0 5.1 2.5 1.2 8.3 7.0 6.7 8.2 6.7 4.6 2.1 IBD remains rare in less-developed tropical countries (Figure 1). Epidemiological studies show that people from tropical countries have the same genetic predisposition to IBD as do people living in industrialized regions. A possible explanation for the regional differences is that living in developed countries exposes people to disease-causing agents absent in lesserdeveloped countries. However, no such causative agent unique to developed countries has been identified. Conversely, perhaps living in lesser-developed countries exposes people to protective factors that prevent IBD. In the mid-1990’s a group of investigators at the University of Iowa hypothesized that exposure to helminths (parasitic worms) protected from IBD (10;11). IBD became prevalent in developed countries as improved hygienic practices decreased helminth exposure. While helminths are nearly eradicated from developed countries, they remain common in lesserdeveloped countries. Figure 1. The global prevalence of IBD. Helminths and the Immune Response (The theoretical basis for TSO®) Helminths are parasitic worms. There are many different species of helminths. People acquire helminths naturally through contact with contaminated food, water or soil. Colonization is most common in children living in tropical areas with poor sanitation. To be successful, helminths must influence the immune system. People with helminths have modulated immune responses characterized by decreased production of IFNγ and diminished delayed-type hypersensitivity responses (12-14). This alteration in the immune response occurs through induction of regulatory cytokines such as IL4, IL10, and TGFβ (13;15). Helminths also alter immune responses in animal experiments showing that this is a general feature of these organisms (12;16;17) (18-20). Helminth-mediated modulation of the immune system can prevent inappropriate responses that cause disease (10;11). There is excellent epidemiologic data showing that helminths protect from development of asthma and atopy (21;22). This may be due to induction of immuno-regulatory IL10 (23). Animal models also show that helminths protect against excessive inflammation. Helminths inhibit onset of autoimmune diabetes in mice (24;25). In murine experimental autoimmune Figure 2. Mice exposed to encephalitis (a model of multiple sclerosis), helminths inhibit helminths are protected from disease (26;27). Importantly, helminths prevent onset of developing TNBS colitis. spontaneous colitis in IL10 deficient mice (10), and protect mice Adapted from Elliott, et.al., Amer. J. and rats from developing severe colitis in response to Physiol. 284:G385, 2003. dinitrobenzene sulfonic acid or trinitrobenzene sulfonic acid (28-30), see Figure 2. Helminths and humans co-evolved. Until the twentieth century, people routinely carried helminths. Our immune systems are adapted for helminths. It is likely that loss of exposure to these organisms has affected our immune systems permitting development of excessive immune responses that cause IBD. The choice of Trichuris suis. As detailed above, there is a strong basis for using helminths as natural immune modulators to treat IBD. Such a treatment strategy requires use of a helminth suited for medicinal application. Some helminths have significant potential to cause disease that hinders their therapeutic use. Others reproduce in ways that could permit inadvertent colonization of others. An medicinal helminth should, 1) produce self-limited colonization in humans, 2) have no potential to cause human disease, 3) not replicate in the host, 4) not be transmissible, and 5) be free of other contaminating agents or organisms. Trichuris suis, the active component in TSO®, meets these requirements. Trichuris suis is the porcine whipworm (Figure 3). It is related to, but distinct from, Trichuris trichiura, the human whipworm (31). T. suis can colonize people but the worms are short-lived and colonization is self-limited. Although many farmers are exposed to T. suis, this helminth does not cause disease in people. T. suis cannot increase their numbers (replicate) in people. T. suis ova require a 3 to 6 week incubation in moist soil to mature. Thus, inadvertent spread to others is highly unlikely. Finally, as described below, T. suis can be isolated and purified free of other agents and organisms. T. suis is acquired by ingesting developed ova. The ova are nearly microscopic measuring 24 µm x 54 µm. Once ingested, the egg hatches to release a single T. suis larva. Over two months, the larvae mature, migrating from the small bowel to the cecum and ascending colon. The body of adult worms is about 1 cm in length. They have a thin hair-like appendage that attaches to the host giving them a “whip-like” appearance. Like most helminths, T. suis cannot mature outside of a host. At Ovamed, we colonize young pigs with T. suis to obtain adult worms. Once the worms are mature, they are removed from the pig, washed, and then grown in the laboratory to obtain ova. Ova then are processed and extensively tested to assure uniformity. Figure 3. Adult Trichuris suis worms T. suis for treatment of IBD. Researchers have performed clinical trials with T. suis to treat Crohn’s disease and ulcerative colitis. They found that T. suis is a safe and effective treatment for IBD (32). This treatment was found safe even in patients using steroids and/or azathioprine/6-mercaptopurine. Figure 4 shows response profiles for IBD patients treated with T. suis ova. In these examples, the patients were treated with a single dose of 2500 ova then observed for 12 to 15 weeks. The patient with Crohn’s disease responded to the first dose with a drop in the Crohn’s Disease Activity Index (CDAI) to below 150. The CDAI is a standard index of clinical symptoms and signs for determining how Crohn’s disease responds to treatments (33). CDAI scores less than 150 indicate clinical remission. After about 8 weeks, the effect waned and the patient relapsed. This likely correlates with the fairly rapid loss of the T. suis in human hosts. The patient began maintenance treatment at week 16 receiving 2500 ova every 3 weeks. With maintenance treatment, the patient went back into remission. The lower chart in Figure 4 shows the response profile of an ulcerative colitis patient treated with T. suis. This study used the Simple Clinical Colitis Activity Index (SCCAI) (34) to measure ulcerative colitis disease activity. The patient with ulcerative colitis received a dose of 2500 ova. The patient improved and did not relapse before entering maintenance treatment. With T. suis maintenance therapy the patient achieved remission. These and other studies have demonstrated that T. suis therapy is safe and effective in IBD. The studies show that T. suis Figure 4. Response profiles of a Crohn’s causes no symptoms as compared to placebo. T. disease patient and an ulcerative colitis suis is safe at doses up to at least 2500 ova patient treated with T. suis ova. Adapted from every 2 weeks. Concurrent immune suppression Summers, et. al., Amer. J. Gastro. 98:2034, 2003 with prednisone, azathioprine, and/or 6mercaptopurine does not hinder response to or safety of T. suis. It takes about 6-8 weeks for most patients to respond to T. suis therapy. Because the helminth is short-lived in people, repeated dosing is required to maintain response. TSO® - INSTRUCTIONS FOR USE TSO® contains viable Trichuris suis ova that are the active agent. TSO® is not metabolized and does not circulate in the body. The T. suis ova are extensively tested for uniformity and safety. TSO® is used for immune-mediated inflammatory conditions like IBD that may result from loss of natural helminth exposure. Response time and use of concomitant medications: Response to ImmNova® can require several weeks. Therefore, TSO® is not indicated for fulminate colitis or conditions that may require urgent surgical correction. TSO® is useful for active or quiescent disease, and can be used in combination with other therapies. TSO® appears safe to use in patients on moderate or low-dose corticosteroids, and/or azathioprine or 6mercaptopurine. TSO has not been extensively tested in patients treated with infliximab (Remicade®), cyclosporin A, or methotrexate. If a patient that requires corticosteroids to remain in remission begins TSO®, it is recommended that they remain on TSO® for 8 weeks before attempting to slowly taper the steroids. Administration: Most patients are started at 500 ova/dose. They receive TSO® orally every 1 to 3 weeks on an “empty stomach”. If the patient has not responded to TSO within 8 weeks (4 treatments) the dose is increased to 1000 ova/dose. The current maximal recommended dose of TSO is 2500 ova every 2 weeks. To take TSO®, gently agitate the vial to disperse the ova. Pour the contents into a disposable cup containing dilute juice or a non-acidic electrolyte re-hydration (sports) drink. Add a small amount of water or juice to the vial, agitate, and pour the contents again into the disposable cup. Avoid mixing TSO® with hot drinks (eg. coffee or tea). Avoid mixing TSO® with alcoholic beverages (eg. beer, wine, or liquor). The patient should drink the contents of the cup then dispose of the cup into a waste container. Do not let others drink from the cup that contains TSO®. Storage and handling: Because TSO® contains living organisms, it must be protected from high temperatures or freezing. Do not expose TSO® to temperatures greater than 40°C. TSO® should be refrigerated at 4° to 10°C. Do not freeze TSO®. It should not be stored below 4°C due to the risk of freezing. Do not place the vials directly on freezer packs or in ice that could be below 0°C. Contraindications and adverse events: TSO® is contra-indicted in patients known to be hypersensitive to Trichuris or compounds made from Trichuris. If an adverse reaction to the agent is suspect, treatment with albendazole (400 mg) will eliminate the agent. Patient acceptability of TSO®: TSO® is a natural way to modify inappropriate mucosal immune responses. Patients are very accepting of the therapy when they understand the principles supporting this treatment. Patients take TSO® orally every 1 to 3 weeks. Patients are relieved to know that they ingest almost microscopic eggs and do not find (see) worms or eggs in the stool. The therapy does not appear to cause side effects or complications. The therapy can be given alone or in conjunction with other standard medications for inflammatory bowel disease. References 1. MacDonald, T.T., P. Hutchings, M.Y. Choy, S. Murch, and A. Cooke. 1990. Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clinical & Experimental Immunology 81:301. 2. Niessner, M. and B.A. Volk. 1995. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR). Clinical & Experimental Immunology 101:428. 3. Fuss, I.J., M. Neurath, M. Boirivant, J.S. Klein, C. de la Motte, S.A. Strong, C. Fiocchi, and W. Strober. 1996. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J.Immunol. 157:1261. 4. Lala, S., Y. Ogura, C. Osborne, S.Y. Hor, A. Bromfield, S. Davies, O. Ogunbiyi, G. Nunez, and S. Keshav. 2003. Crohn's disease and the NOD2 gene: a role for paneth cells. Gastroenterology 125:47. 5. Inohara, N., Y. Ogura, A. Fontalba, O. Gutierrez, F. Pons, J. Crespo, K. Fukase, S. Inamura, S. Kusumoto, M. Hashimoto, S.J. Foster, A.P. Moran, J.L. Fernandez-Luna, and G. Nunez. 2003. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J.Biol.Chem. 278:5509. 6. Lapidus, A. 2001. The changing epidemiology of inflammatory bowel diseases. Acta Gastroenterologica Belgica 64:155. 7. Loftus, E.V.J. and W.J. Sandborn. 2002. Epidemiology of inflammatory bowel disease. Gastroenterology Clinics of North America 31:1. 8. Yang, S.K., W.S. Hong, Y.I. Min, H.Y. Kim, J.Y. Yoo, P.L. Rhee, J.C. Rhee, D.K. Chang, I.S. Song, S.A. Jung, E.B. Park, H.M. Yoo, D.K. Lee, and Y.K. Kim. 2000. Incidence and prevalence of ulcerative colitis in the SongpaKangdong District, Seoul, Korea, 1986-1997. Journal of Gastroenterology & Hepatology 15:1037. 9. Bach, J.F. 2002. The effect of infections on susceptibility to autoimmune and allergic diseases. New England Journal of Medicine 347:911. 10. Elliott, D.E., J.F.J. Urban, C.K. Argo, and J.V. Weinstock. 2000. Does the failure to acquire helminthic parasites predispose to Crohn's disease? FASEB Journal 14:1848. 11. Weinstock, J.V., R.W. Summers, and D.E. Elliott. 2004. Helminths and harmony. Gut 53:7. 12. Sabin, E.A., M.I. Araujo, E.M. Carvalho, and E.J. Pearce. 1996. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. Journal of Infectious Diseases 173:269. 13. Bentwich, Z., Z. Weisman, C. Moroz, S. Bar-Yehuda, and A. Kalinkovich. 1996. Immune dysregulation in Ethiopian immigrants in Israel: relevance to helminth infections? Clinical & Experimental Immunology 103:239. 14. Borkow, G., Q. Leng, Z. Weisman, M. Stein, N. Galai, A. Kalinkovich, and Z. Bentwich. 2000. Chronic immune activation associated with intestinal helminth infections results in impaired signal transduction and anergy. Journal of Clinical Investigation 106:1053. 15. Doetze, A., J. Satoguina, G. Burchard, T. Rau, C. Loliger, B. Fleischer, and A. Hoerauf. 2000. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by T(h)3/T(r)1-type cytokines IL-10 and transforming growth factor-beta but not by a T(h)1 to T(h)2 shift. International Immunology 12:623. 16. Kullberg, M.C., E.J. Pearce, S.E. Hieny, A. Sher, and J.A. Berzofsky. 1992. Infection with Schistosoma mansoni alters Th1/Th2 cytokine responses to a non-parasite antigen. J.Immunol. 148:3264. 17. Pearlman, E., J.W. Kazura, F.E.J. Hazlett, and W.H. Boom. 1993. Modulation of murine cytokine responses to mycobacterial antigens by helminth-induced T helper 2 cell responses. J.Immunol. 151:4857. 18. Sacco, R., M. Hagen, M. Sandor, J.V. Weinstock, and R.G. Lynch. 2002. Established T(H1) granulomatous responses induced by active Mycobacterium avium infection switch to T(H2) following challenge with Schistosoma mansoni. Clinical Immunology 104:274. 19. Ledingham, D.L., V.C. McAlister, H.N. Ehigiator, C. Giacomantonio, M. Theal, and T.D. Lee. 1996. Prolongation of rat kidney allograft survival by nematodes. Transplantation 61:184. 20. Kuroda, E., Y. Yoshida, S.B. En, and U. Yamashita. 2001. Suppression of macrophage interleukin-12 and tumour necrosis factor-alpha production in mice infected with Toxocara canis. Parasite Immunology 23:305. 21. van den Biggelaar, A.H., C. Lopuhaa, R. van Ree, J.S. van der Zee, J. Jans, A. Hoek, B. Migombet, S. Borrmann, D. Luckner, P.G. Kremsner, and M. Yazdanbakhsh. 2001. The prevalence of parasite infestation and house dust mite sensitization in Gabonese schoolchildren. International Archives of Allergy & Immunology 126:231. 22. Yazdanbakhsh, M., P.G. Kremsner, and R. van Ree. 2002. Allergy, parasites, and the hygiene hypothesis. Science 296:490. 23. van den Biggelaar, A.H., R. van Ree, L.C. Rodrigues, B. Lell, A.M. Deelder, P.G. Kremsner, and M. Yazdanbakhsh. 2000. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet 356:1723. 24. Zaccone, P., Z. Fehervari, F.M. Jones, S. Sidobre, M. Kronenberg, D.W. Dunne, and A. Cooke. 2003. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur.J.Immunol. 33:1439. 25. Cooke, A., P. Tonks, F.M. Jones, H. O'Shea, P. Hutchings, A.J. Fulford, and D.W. Dunne. 1999. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunology 21 :169. 26. Sewell, D., Z. Qing, E. Reinke, D. Elliot, J. Weinstock, M. Sandor, and Z. Fabry. 2003. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. International Immunology 15:59. 27. La Flamme, A.C., K. Ruddenklau, and B.T. Backstrom. 2003. Schistosomiasis decreases central nervous system inflammation and alters the progression of experimental autoimmune encephalomyelitis. Infection & Immunity 71:4996. 28. Elliott, D., J. Li, A. Blum, A. Metwali, K. Qadir, J.F.J. Urban, and Weinstock J.V. 2003. Exposure to Schistosome Eggs protects mice from TNBSinduced colitis. American Journal of Physiology 284:G385. 29. Khan, W.I., P.A. Blennerhasset, A.K. Varghese, S.K. Chowdhury, P. Omsted, Y. Deng, and S.M. Collins. 2002. Intestinal nematode infection ameliorates experimental colitis in mice. Infection & Immunity 70:5931. 30. Moreels, T.G., R.J. Nieuwendijk, J.G. De Man, B.Y. De Winter, A.G. Herman, E.A. Van Marck, and P.A. Pelckmans. 2004. Concurrent infection with Schistosoma mansoni attenuates inflammation induced changes in colonic morphology, cytokine levels, and smooth muscle contractility of trinitrobenzene sulphonic acid induced colitis in rats. Gut 53:99. 31. Beer, R.J. 1976. The relationship between Trichuris trichiura (Linnaeus 1758) of man and Trichuris suis (Schrank 1788) of the pig. Research in Veterinary Science 20:47. 32. Summers, R.W., D.E. Elliott, K. Qadir, J.F.J. Urban, R. Thompson, and J.V. Weinstock. 2003. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. American Journal of Gastroenterology 98:2034. 33. Best, W.R., J.M. Becktel, J.W. Singleton, and F. Kern, Jr. 1976. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology 70:439. 34. Walmsley, R.S., R.C. Ayres, R.E. Pounder, and R.N. Allan. 1998. A simple clinical colitis activity index. Gut 43:29.