* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Full Text in English - Health Science Journals: Indonesia
Innate immune system wikipedia , lookup
Rheumatic fever wikipedia , lookup
Ulcerative colitis wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Human leukocyte antigen wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Kawasaki disease wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Pathophysiology of multiple sclerosis wikipedia , lookup
Autoimmunity wikipedia , lookup
Molecular mimicry wikipedia , lookup
IgA nephropathy wikipedia , lookup
Ankylosing spondylitis wikipedia , lookup
Behçet's disease wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Germ theory of disease wikipedia , lookup
Globalization and disease wikipedia , lookup
Inflammatory bowel disease wikipedia , lookup
Rheumatoid arthritis wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Neuromyelitis optica wikipedia , lookup
Sjögren syndrome wikipedia , lookup
Multiple sclerosis research wikipedia , lookup
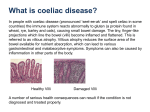
Tinjauan Pustaka Coeliac Disease: Focus on Multisteps Immunopathogenesis and Their Correspond Clinical Investigations I Gede Yasa Asmara Faculty of Medicine, University of Mataram Abstract: Coeliac disease is a chronic inflammatory intestinal disease as a result of gluten hypersensitivity. It affects 1 in 200 people mainly in Western countries. There are four main pathogenic steps of coeliac disease which correspond to each specific clinical investigation; The first begin with the introduction of gluten into the intestinal mucosa which leads to the development of antigliadin antibodies. Specific gluten peptides undergo deamidation by tissue transglutaminase in the lamina propria of the small gut. Serologically, anti endomysial and anti-tissue transglutaminase antibodies can be detected in this phase. Next, local macrophages which act as professional antigen presenting cells (APCs) processed the deaminated peptides and present it to the T cells via specific MHC type II. It is therefore molecular technique is able to detect the present of particular HLA allele of the MHC type II. Finally, small bowel mucosal and villi destruction occur and can be examined using endoscopy and biopsy. The last method of investigations remains the gold standard of diagnosis of coeliac disease. An improved understanding of the immunopathogenesis of coeliac disease is likely to lead to the improvement of diagnostic ability and the development of novel strategies for the treatment of coeliac disease. Keywords: Coeliac disease, immunopathogenesis, clinical investigations, diagnosis Maj Kedokt Indon, Volum: 58, Nomor: 12, Desember 2008 531 Focus on Multisteps Immunopathogenesis and Their Correspond Clinical Investigations Coeliac Disease: Focus on Multisteps Immunopathogenesis and Their Correspond Clinical Investigations I Gede Yasa Asmara Faculty of Medicine, University of Mataram Abstrak: Penyakit soeliak adalah penyakit inflamasi kronik pada usus halus yang diakibatkan oleh hipersensitivitas terhadap produk tepung. Penyakit ini mengenai 1 dari 200 orang terutama di negara barat. Ada 4 tahapan penting dalam patogenesis penyakit seliak yang setiap tahapan berkorelasi dengan pemeriksaan penunjang tertentu. Pertama, pemeriksaan antibodi anti-gliadin dapat dilakukan pada fase awal ketika mukosa usus halus terpapar oleh produk tepung. Kedua, antibodi anti-endomisial dan anti transglutaminase jaringan dapat dideteksi dalam darah pada fase deamidasi protein gluten di lapisan lamina propria usus halus. Ketiga, protein terdeamidasi akan diproses dan dipresentasikan oleh makrofag jaringan kepada sel T melalui komplek major histokompatibilitas tipe II. Pada fase ini pemeriksaan molekuler dengan menggunakan PCR dapat mendeteksi adanya HLA alel tertentu pada pasien. Pada fase terakhir saat mukosa dan vili usus halus telah mengalami kerusakan akibat respon imun, pemeriksaan endoskopi dan biopsi dapat dilakukan. Pemeriksaan terakhir masih merupakan baku emas diagnosis penyakit seliak. Pemahaman yang baik dalam proses imunopatogenesis penyakit ini sangat penting dalam menentukan pemeriksaan penunjang yang diperlukan dalam diagnosis dan pengembangan terapi terbaru. Kata kunci: penyakit seliak, imunopatogenesis, pemeriksaan penunjang, diagnosis Introduction In some pathogenesis of diseases, environmental factors, genetics, as well as host response form complex interplay giving rise to the clinical signs and symptoms. Some immunological disorders such as rheumatoid arthritis, systemic lupus erythematosus, irritable bowel syndrome and coeliac disease also fit with this concept. Coeliac Disease (CD) which will be discussed here is chronic intestinal inflammatory disorder.1 It occurs as a result of environmental exposure (wheat gluten) to genetically susceptible individual.2 It is thought to be organ specific autoimmune disease since autoantibody is produced in its pathogenic process. Since CD was firstly described by Samuel Gee in 1888,3 there has been a lot of research exploring its immuno-pathogenesis, relation to other diseases and therapeutic interventions. However, some molecular mechanisms of CD remain unresolved. Epidemiology Coeliac disease or gluten sensitive enteropathy is quite common, affecting 1 in 250 people in the world.1 It mainly occurs in Western countries such as Europe and some part of North America. For this reason, it is called a disease of Caucasians.4 The incidence of CD is even higher based on 532 serologic screening, where it is found in 1 in 200 populations.2 Other studies revealed that approximately 1% of population suffers from CD.5-7 This condition can be diagnosed either in early childhood or late during the adulthood, with peak age of diagnosis at forth or fifth decade of life. Women are more likely to get CD, accounted for 3:1,6 whereas the equal proportion may be the case in childhood.7 It seems that CD is under-diagnosed because of considerable number of silent disease with mild pathogenic mucosa changes. This has been attributed to physician delay in diagnosis instead of patient’s delay in seeking health care.6 CD is acquired within the family and a good example of disease related to HLA alleles.4 CD is likely to occur in 10% of first degree of relatives. Concordance in identical twins is around 70%.1,4,6 Several studies reported that HLA-DQ2 (DQA1*0501/DQB1*0201) and in some extent to HLA-DQ8 (DQA1*0301/DQB1 *0302) associated with CD.1-9 Roughly 95% of patients carry both HLA alleles, indicating CD is unlikely to develop in person with absent of these HLA alleles.5 In addition, other genetic factors such as abnormality in chromosome 5, 6, 11 and 19 have been investigated.1,5,8 However, direct relation is not clearly defined yet. There are certain people who have high risk for developing CD and serologic testing is strongly recommended. Maj Kedokt Indon, Volum: 58, Nomor: 12, Desember 2008 Focus on Multisteps Immunopathogenesis and Their Correspond Clinical Investigations For instance, first degree or may be second degree relatives of affected individual, patient with type 1 diabetes, Down or Turner syndrome, selective IgA deficiency and Sjõgren syndrome.3,9,10 Regarding environmental factor, gluten (protein from wheat, barley and rye) has been known as the major factor eliciting sequential immune response in small intestine in CD.3 Moreover, a large epidemiological study in Sweden reported that lack of breast feeding, a large amount of gluten in the infant formula and >3 infections during childhood play an important role increasing the risk of CD.6 It seems that breastfeeding delays the onset and alters the clinical presentation of CD in children.6,11 Clinical Presentation CD is classified based on its clinical features into two categories namely symptomatic/classical/typical and asymptomatic/atypical/silent.6 The presence of diarrhoea with or without malabsorption is characterised the classical disease, whereas the silent disease lacks those symptoms.1,9,10 There are slightly different signs and symptoms between children and adult. In childhood, patients could present diarrhoea, short stature, failure to thrive, anaemia and sometimes constipation.11 In contrast, weight loss, diarrhoea and anaemia are still the common features in adulthood.10 Some other symptoms that have been reported are osteoporosis, infertility, a variety of neurological disorders, dilated cardiomyopathy and myocarditis.3,10,12 CD is also associated with other autoimmune diseases, immunodeficiency and malignancy. Serum IgA deficiency has been reported to have at least ten fold increased risk of CD.3 Similarly, 1.7-2.6% of coeliac patient is also IgA- deficient.1 It becomes important in the interpretation when IgA based serologic tests are employed to establish the diagnosis. The prevalence of CD in Insulin-Dependent Diabetes Mellitus (IDDM) has been reported to be 2-7%.3 In addition, CD increased risk for malignancy or mortality of two fold or greater, particularly intestinal T-cell lymphoma.3,12 Immunopathogenesis As mentioned earlier, the pathogenesis of CD is involving environmental factor (gluten from wheat), genetic susceptibility (HLA-DQ2 or -DQ8) and immune response (T cells activation in the lamina propria).2,13,14 CD is characterised by hypersensitivity to gluten.3 There are four main steps in the development of CD, i.e., introduction of gluten to intestinal mucosa, deamidation of gluten by endogenous tissue transglutaminase enzyme (tTG), presentation of toxic gluten peptide by Antigen Presenting Cells (APCs) bearing HLADQ2 or -DQ8 and destruction of intestinal mucosa (villus atrophy and crypt hyperplasia). Gluten is the term for the storage protein of wheat, which made up of gliadin and glutenin. In general, wheat gluten Maj Kedokt Indon, Volum: 58, Nomor: 12, Desember 2008 protein consists mainly of proline and glutamine residues.2,4,5 Gliadin, an alcohol soluble fraction of gluten is toxic, inducing activation CD4 T cell in intestinal lamina propria.2,15 Why only gluten elicits this harmful type of immune response is still unclear. However, some evidence indicated that gluten might have an additional activity inducing T cell responses such as increasing HLA-DR expression and Intercellular Cell Adhesion Molecules (ICAM).2,15 Another reason could be gluten peptide is absorbed in higher concentration than peptides of other proteins.2 Thus their level in lamina propria is enough to be recognised by CD4+T cells. In addition, gliadin which has been described as 33mer sequence of α gliadin is resistant to intestinal peptidase such as pepsin and chymotrypsin. It is because gastric and pancreatic enzymes lack post-proline cleaving activity.5,16 Hence, it is able to reach lamina propria and stimulate T cells. At this first pathogenic step, an evidence of antigliadin antibody could be obtained and useful for diagnosis CD. During the first response of innate immunity against toxic wheat gluten, this peptide should be recognised by intestinal APCs and presented to CD4 T cells. At this point, APCs bearing HLA-DQ2 or -DQ8 play an important role in efficiently present the peptide to CD4 T cells. In order to be presented efficiently, gluten needs to be deamidated by so called tTG.1,17,18 Deamidation is modification of glutamine to glutamic acid leading to a mass increase of 1 Da and negatively charge molecules.2 Deamidation of gluten occurs just before gluten is endocytosed by APCs. Tissue transglutaminase (tTG) is a Ca2+ dependent enzyme which is ubiquitous just beneath the epithelium in the gut wall as well as other tissues and organs.2,18 At this second pathogenic step, antibody to endomysium (tTG as an auto antigen) could be detected as a very good marker for diagnosing CD. As for following pathogenic sequence, HLA-DQ2 or DQ8 has prefential to bind peptides with negatively charge amino acids present in key positions. APCs with HLA-DQ2 or -DQ8 then efficiently present this toxic peptide to CD4 T cells.8 As a result of the CD4 T cells activation, cytokines such as interferon gamma (IFNγ) and Tumour Necrosis Alfa (TNFα) are produced.4,16,19 TNFα activates stromal cells to produce Keratinocyte Growth Factor (KGF), and KGF causes epithelial proliferation and crypt cell hyperplasia. Furthermore, TNFα and IFNγ can jointly have a direct cytotoxic effect on intestinal epithelial cells. In conclusion, those cytokines are responsible for not only epithelial cell destruction but also crypt cells proliferation,4 which classically characterise the last pathogenic step of CD (villous atrophy and crypt cell hyperplasia).9,12 Interestingly, gliadin itself also has ability to induce the production of IL-15 by APCs. IL-15 is then induce the over expression of a stress molecule, MICA, on the surface of enterocytes and up regulate NKG2D receptor on intraepithelial lymphocytes (IEL).7,20 The interactions between these two molecules lead to direct enterocytes killing 533 Focus on Multisteps Immunopathogenesis and Their Correspond Clinical Investigations (apoptosis). Another direct effect of gliadin is the increase of intestinal permeability via release of zonulin and effects on intracellular tight junction.7,15 The net result is more uptake of gliadin to the lamina propria. The presence of serum autoantibodies directed against gliadin, tTG and endomysium is very helpful in establishing the diagnosis of CD. Moreover, tTG antibody is a specific marker for active disease.3 Nevertheless, the mechanism of antibody formation is still a mystery. One possible hypothesis could be complex between gluten peptide and tTG act in a hapten carrier like fashion.2 The complex is then taken up by tTG specific B cell and after intracellular degradation, gluten-derived cells is presented to T cell through HLADQ2 or -DQ8. The activated T cells, in turn, provide help to the B cell in producing specific antibodies.4,5 Clinical Investigation In correlation to the four steps of immunopathogenesis in CD, there are several laboratory examinations which can be performed corresponding to each step. In the first step in which wheat gliadin peptide is introduced to intestinal mucosa, antigliadin antibody (AGA) can be detected. Both IgA and IgG AGA were detected using indirect immunofluorescence.3 Subsequently, the introduction of Enzyme Linked Immunosorbent Assay (ELISA) made diagnosis of CD more rapidly and conveniently performed. However, IgA and IgG AGA have a lower sensitivity and specificity than IgA Endomysial Antibody (EMA) assay and tTG IgA assay, especially in adult and children over the age of two years.3,7 On the contrary, in children under the age of two years, IgG AGA may still useful since serum IgA slowly raise in children toward adulthood. Similarly, in patient with selective IgA deficiency, IgG AGA may also beneficial.3,9,11 The second step of pathogenesis of CD is deamidation of gliadin by tTG. In this stage, both IgA EMA and IgA tTG antibodies play a pivotal role. Since EMA antibody assay was introduced by Chorzelski et al in 1980s,3 it has been widely used in part of diagnosing CD. It has very high specificity (99%) and sensitivity (90%).1,3,7 It was performed initially by indirect immunofluorescence with the lower third of monkey oesophagus as a tissue substrate. However, the test is labour intensive and qualitative, this requires money, time and expertise to perform.3,7 Moreover, due to ethics and cost, human umbilical cord has replaced monkey oesophagus as a substrate. In addition, it has been revealed that the human umbilical assay demonstrated a better interlaboratory reproducibility.3 IgA EMA antibody assay can be used in the monitoring of CD, whereas IgG EMA could not. It because IgG EMA still recover in the serum despite gluten-free diet.21 The development of ELISA also brings about measurement of tTG which is the autoantigen of EMA. Although tTG is not necessary a single antigen for EMA, this assay may still have a comparable sensitivity and specificity to the 534 EMA antibody test.3,16 tTG antibody assay is easier, quicker and quantitative so that it is superior than EMA antibody test.7 Some previous studies used guinea pig liver tTG (gpltTG) as substrate, whereas now it has been replaced by purified erythrocyte and recombinant human tTG. Furthermore, human tTG has a higher specificity than gpl-tTG even where purified gpl-tTG was used. 3 Serotype based association study revealed that the association of HLA-DQ2 and -DQ8 in CD. A European study reported than 90% coeliac patient possessed HLA-DQ2 and the rest possessed HLA-DQ8.7 This has lead to the development of HLA-DQ typing, which is performed using PCR with sequence specific primer. Some studies mentioned the involvement of non HLA-DQ alleles such as HLA-DR3, -DR5 and -DR7 in the pathogenesis of coeliac disease.4,8 However, the evidence is not conclusive. Since 40% of general population has HLA-DQ2 or -DQ8, HLA-DQ typing appears to be a necessary but not sufficient, factor in CD diagnosis.6,9 Therefore, in the case where the diagnosis of coeliac disease is unclear, screening for patient’s family member and excluding coeliac disease when the patient are already on a gluten free diet, HLA-DQ typing may be essential.9 The last step of CD pathogenesis is the destruction of intestinal mucosa as a result of chronic inflammatory response. In this stage, biopsy remains the gold standard in the diagnosis of CD. Since the nature of villous atrophy is patchy, multiple biopsies are necessary which focus on the descending duodenum rather than the more distal intestine.9,12 The spectrum of histologic changes in CD is quite wide from mild to severe villous atrophy. Marsh9 classified histopathology change in coeliac disease into three stages namely:9 Marsh I is characterised by normal villous architecture with IEL (>30 lymphocytes per 100 enterocytes); Marsh II is recognised as both IEL and crypt hypertrophy; Marsh III includes the evidence of moderate to severe reduction in villous height. Diagnosis Diagnosis of CD has inproved as the development of serologic testing as well as endoscopic and biopsy procedures. In patients who display classical disease, high index of clinical suspicion is immediately established. In contrast, screening with serologic testing is required to find out silent cases. The use of IgA and IgG EMA and tTG are recommended in screening CD. However, duodenal biopsy should be subsequently performed in serologic positive patients.21 In few cases, coeliac patients may be derived from incidental endoscopic procedures which are performed because of other diseases. The diagnosis of coeliac disease depend on demonstration of the typical biopsy changes of untreated coeliac disease in the small intestinal mucosa and improvement with dietary gluten restriction.9,10 In a rare case where a strong suspicion of the condition exists but in which all test are negative, a rectal gluten chalMaj Kedokt Indon, Volum: 58, Nomor: 12, Desember 2008 Focus on Multisteps Immunopathogenesis and Their Correspond Clinical Investigations lenge may yield a positive result.9 It is based on the fact that the mucosal immune systems involve the rectal as well as the small bowel mucosa. Additionally, a rectal gluten challenge is an easy office procedure and a direct provocation test of gluten sensitivity while other tests are indirect.9 Treatment The current treatment for CD is a gluten free diet for a long life.12,19,22 In this diet, any food product containing wheat, barley and rye must be avoided. It is unclear whether oat is toxic to the patients. In severe cases, patient do require admission, replacement of fluids and electrolytes and occasionally, steroid.12,19 Tabel 1. Main Pathogenic Steps of Coeliac Disease and Their Correspond Test Main pathogenic steps Clinical investigations Ingestions of gluten-containing food Deamidation of gluten peptides by tissue transglutaminase Anti gliadin antibodies Anti-endomysial and antitissue transglutaminase anti bodies HLA-DQ typing Presentation of modified peptides by HLA class II Induction of autoimmune mucosal injury Small bowel biopsy Education of the patient and his/her supportive household is inevitably important. Patient should know the fluors and grain that are naturally gluten free such as rice, corn, potato, chestnut, fluor, millet as well as buckwheat.10 Since fluor from wheat is usually fortified with iron, thiamine, riboflavin and niacin. It is recommended that gluten free diet is supplemented by those vitamins and trace elements.6,10 There are several novel treatments of CD being developed based on its pathophysiologic mechanism. Since gliadin which is the 33mer is toxic, enzyme supplementation of bacterial prophyl endopeptidase is developed to degrade the T cells stimulatory peptide.7 Alternatively, producing wheat with absent or reduced immunogenicity by selective breeding or genetic modification is also on research.7,22 Another strategy is also targeting tTG, zonulin and HLA peptide interaction.7,19 However, none of them is in clinical use yet. Summary Coeliac disease is a good example of autoimmune disorder where environmental and genetic factors play an equal role in its pathogenesis. There has been a lot of evidence revealing the multisteps of host immune response against wheat gluten resulting in the disease. We all now know that each step can be demonstated by spesific clinical investigations leading to the diagnosis of coeliac disease. Moreover, a good understanding of the immunopathogenesis of coeliac disease might help the clinician in deciding the correct intervention. However, there is still a major challenge ahead in transfering this knowledge into practical benefit for the patients. Reference 1. 2. Future 1. Sequence Evens in the Immunopathogenesis of Coeliac disease17 1. Gluten is digested to yield peptides, which are transported into mucosa; 2. Key glutamine residues are deamidated by tissue transglutaminase; 3. Epitope processing and presentation with DQ2 by dendritic cells; 4. Gluten-sensitive T cells recognize epitope and are stimulated; 5. Lamina pro pria lymphocytes proliferate and recruit cellular infiltrate; 6. CD8 T cells with cytotoxic markers increase in mucosa; 7. Fibroblasts are activated and produce metalloproteinases to degrade matrix; 8. Plasma cells pro duce disease-specific coeliac antibodies; 9. The role of primitive intra-epithelial lymphocytes remains unclear. Maj Kedokt Indon, Volum: 58, Nomor: 12, Desember 2008 Green PHR, Jabri B. Coeliac disease. Lancet. 2003;362:383-91. Sollid LM. Coeliac disease: dissecting a complex inflammatory disorder. Nature reviews. 2002;2:647-55. 3. Wong RC. Steele RH. Reeves GE. Wilson RJ. Pink A. Adelstein S. Antibody and genetic testing in coeliac disease. Pathology. 2003;35(4):285-30. 4. Sollid LM. Molecular basis of celiac disease. Ann Rev Immunol. 2000;18:53-81. 5. Stepniak D, Koning F. Celiac disease-sandwiched between innate and adaptive immunity. Human Immunology. 2006;67:460-8. 6. Green PHR, Jabri B. Coeliac disease. Ann Rev Med. 2006;57:20721. 7. van Heel DA, West J. Recent advances in coeliac disease. Gut. 2006;55:1037-46. 8. van Heel DA, Hunt K, Greco L, Wijmenga C. Genetic in coeliac disease. Best Practice & Research in Clinical Gastroenterology. 2005;19(3):323-39. 9. Green PHR, Rostami K, Marsh MN. Diagnosis of coeliac disease. Best Practice & Research in Clinical Gastroenterology. 2005;19(3):389-400. 10. Freeman H, Lemoyne M, Pare P. Coeliac disease. Best Practice & Research in Clinical Gastroenterology. 2002;16(1):37-49. 11. Fasano A, Catassi C. Coeliac disease in children. Best Practice & Research in Clinical Gastroenterology. 2005;19(3):467-78. 12. Ciclitira PJ, Johnson MW, Dewar DH, Ellis HJ. The pathogenesis of coeliac disease. Molecular Aspects of Medicine. 2005;26:42158. 535