* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Optical Measurements
Survey
Document related concepts
Cross section (physics) wikipedia , lookup
Electron scattering wikipedia , lookup
Heat transfer physics wikipedia , lookup
Chemical imaging wikipedia , lookup
Atomic absorption spectroscopy wikipedia , lookup
Particle-size distribution wikipedia , lookup
Photoelectric effect wikipedia , lookup
Photoacoustic effect wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Ellipsometry wikipedia , lookup
Astronomical spectroscopy wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Vibrational analysis with scanning probe microscopy wikipedia , lookup
Transcript
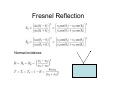
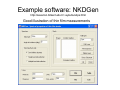
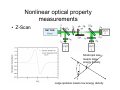
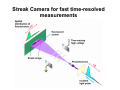
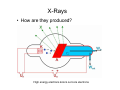
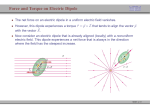
Optical Measurements MSN506 notes note: Most of the information in this presentation is collected off the web for educational purposes. Overview • Remember some relevant parts of EM theory • Survey of some optical techniques – Large number of variants and different techniques are present – It is not possible to cover all of them – Those that may be related to nanomaterial characterization are highlighted Why optical measurements • Optical properties of materials can be naturally measured with optical measurements (i.e. measurements that involve light generation or scattering) • Optical properties can be used to determine structural or other physical properties • Generally non-destructive Interaction of light with matter • Light is an electromagnetic wave and electric (or magnetic fields) interact with charge • For light to interact with matter, generally carriers must be present (which they generally are) • Light can interact with bound or free carriers • Interaction can be non-resonant or resonant (i.e. frequency or wavelength of light can coincide with a characteristic oscillation frequency of the sample) The dipole moment • Emission (and absorption or scattering) of light by the presence of a carrier is strongly affected by the dipole moment and its properties Oscillating dipole Proportional to the dipole moment Power radiated by a classical dipole Dipole moment (Quantum Mech.) • The dipole moment can be calculated for classical charge distributions 1D potential well pd G = er Time dependent dipole during a transition between energy levels • During a transition, dipole oscillates with a frequency =ω = ΔE A B ++ A+B A-B Light can be generated Power radiated by a classical dipole - - + Absorption can also be resonant (this can be understood via time reversal symmetry of electromagnetic waves) Dipoles and refractive indices Refractive index as a function of frequency is determined by the AC permitivity Dipoles and refractive indices Refractive index as a function of frequency is determined by the AC permitivity Sometimes negative, depends on convention Generally does not depend on frequency at optical frequencies n* = n + i · k (n + ik)2 = ε' + i · ε'' ⎛ 2 ⎜ ⎝ ⎛ ⎝ ⎛ 2 ⎜ ⎝ ⎛ ⎝ 1 n2 = 1 κ2 = ε' 2 + ε'' 2 ε' 2 + ε'' 2 ⎞ ⎠ ⎞ ⎠ + ε' 2 – ε' 2 ⎞ ⎟ ⎠ ⎞ ⎟ ⎠ Oscillator model Damping factor Classical charge oscillator Solution (can be used to calculate polarization) Lorentzian lineshape Absorbed Energy Oscillator strength can be calculated through the transition matrix element for two given levels Oscillator strength Example Single oscillator Multiple oscillators Silicon Density of oscillators Strength of oscillators Absorption due to an oscillator Single oscillator Absorption coefficient Density of oscillators Strength of oscillators Classical oscillator model is intuitive for absorption measurements Example: Atomic spectra Absorption lines characteristic for each atom The Monochromator Can be used to select a certain wavelength in a beam of white light Diffraction grating Multiple orders can be observed depending on the period and wavelength Orders will repeat especially if the grating period is large Blazed at an angle for higher efficiency Summary of optical measurements • Thin films: absorption, reflection, transmission of thin films, Ellisometry, refractive index models, applications, FTIR • Atomic absorption measurements • Light scattering measurements: Raman Scattering, Dynamic light scattering • Nonlinear property measurements • Photoluminescence • Pump/Probe experiments • Diffraction, X-ray Diffraction Thin Films • A material is deposited (or coated) uniformly on a substrate. • Measuring optical propeties of the film we can learn a lot about the material • Semiconductors • Band gap, absorption, refractive index, impurities, defects etc. • Nanoparticles • Size distribution, crystallinity etc. • Organic layers • Molecules • Requires modelling of the measurement scheme Thin film measurements incident substrate transmitted reflected absorbed Film of material to be characterized Support substrate of known optical properties Incident Power = Reflected + Absorbed + Transmitted Powers Fresnel Reflection Normal incidence Thin Film Reflection Transmittance If the substrate is transparent to some degree in the wavelength range of interest, transmission measurements can be used to determine the optical constants. Simple formulas are available for restricted cases 1 T Transmittance Reflection at an angle The Ellipsometer • Absolute quantities are always hard to measure, and most of the time inaccurate • Making reflection measurements at two different polarizations quickly one after another can yield better results The Ellipsometer Less sensitive to intensity fluctuations The Ellipsometer The Ellipsometer Porosity as well Modelling for Ellipsometry • Refractive index models (semiempirical or empirical) • Surface and interface roughness • Composite material models, porosity • Layered materials and gradients • A lot of complexity… • Multiple models can produce similar results • One solution is Variable Angle Spec. Ellips. • Experience helps a lot in modelling Refractive index models • • • • Cauchy Sellmeier Other Models Effective medium models (particularly important for composite materials) Example software: NKDGen http://www.fen.bilkent.edu.tr/~aykutlu/elips.html Good illustration of thin film measurements Example: Material Characterization using transmission data Bulk Absorption Measurements Example: Atomic Absorption Spectrometer Specialized elemental lamp for different atoms Burn the sample and analyze absorption of the flame! Scattering Measurements • Light (generaly a laser) is incident upon the sample • Interaction of sample and light generates modulation of light frequency, spatial distribution and/or time Raman Scattering • observed by C.V. Raman 1928 • Received Nobel price in 1930 Electronic vibration excited by external monochromatic light Mechanical vibration excited by phonons • If these are coupled by a nonlinear interaction, sum and difference frequencies of light can be generated • Light then carries information about the phonon density • Scattered light intensity is very weak compared to original Energy Level Picture • Selection Rules: Polariation • Raman Active modes • Requires a sharp clean light source and a high resolution monochromator Example applications Chemistry: Molecular vibrations Example applications: Bulk Crystals Different structures all at once! SiOx: GeSiO 120 sccm Silicon 1 o 1000 C o 900 C o 800 C o 700 C o 600 C Intensity (a.u) 2 Type VII ta= 2 hours 1 5 SiGe alloy formation 0 300 350 400 450 -1 Frequency shift (cm ) 500 Example:Low-resolution Raman Spectroscopy • Still good for material recognition Low resolution Raman spectrum of 1:1:1 mixture of ethanol, 2propanol, and 2-methyl.2-propanol. Variants • Surface Enhanced Raman Spec. – Plasmonic effects enhance the Raman signal – Single molecule sensitivity! • Waveguide Raman – Multiple (large number) of interactions of laser with sample film enhances the signal – Submonolayer sensitivity • Micro Raman and Tip Enhanced Raman can be used for high spatial resolution FTIR: Fourier Transform Infra Red Spectroscopy • Interferometer has periodic resonance condition • This is used as an advantage in FTIR FTIR principle FTIR Operation FTIR Operation FTIR Operation FTIR bands FTIR summary • Characteristic Frequencies for certain bonds • Dipole moment different for each bond type, intensity varies • Density of bonds affect intensity • Frequency of vibration affected by film stress • Overtones (harmonics) possible • … to get accurate quantitative information meticulous analysis is needed Dynamic Light Scattering • 1. 2. 3. 4. 5. 6. 7. 8. Theory of Operation A beam of monocromatic light is directed through a sample and the fluctuation of the intensity of scattered light by the molecules is analyzed by an Avalanche Photo Diode. The Avalanche Photo Diode then sends electrical pulses to the Digital Signal Processor which counts the number of photons detected in each successive time sample. The frequency spectrum of this signal is determined by the mathematical technique known as autocorrelation. The similarity between the signal wave form and a slightly time delayed copy of itself is determined by multiplying the two wave forms together and then summing to give the autocorrelation function. From this the Translation Diffusion Coefficient, DT can be calculated by performing a nonlinear least-squares fit of the autocorrelation coefficients to an exponential decay. Under the assumption of Brownian Motion and that the molecules in solution are spheres, the Hydrodynamic Radius, RH can be calculated by using Stokes’ Equation. RH = kbT / 6πηDT kb = Boltzman’s Constant T = Absolute Temperature in Kelvin η = Solvent Viscosity Can also estimate the Molecular Weight from the RH and the sample temperature using a standard curve of Molecular Weight vs. RH of globular proteins. The instruments assumes the sample fits a standard monomodal gaussian type distribution (monodisperse) and through a monomodal curve fit, determines the uniformity of the sizes of the species in solution. If the sample is found to be polydisperse or nonmonomodal, the user can use the software to resolve a bimodal size distribution (bimodal analysis). Dynamic Light Scattering Measures size distributions of colloidal (nano) particles in a fluid medium Dynamic Light Scattering 20 nm beads 64 nm beads Nonlinear optical property measurements • Z-Scan Small spot size means large energy density Large spotsize means low energy density Photoluminescence • Measures radiative decay properties of optically generated carriers Photoluminescence Photoluminescence • • • • • Temperature dependent Time resolved Resonant excitation Pump power dependent Polarization dependent • By modelling carrier absorption, relaxation and emission we can estimate sample properties Streak Camera for fast time-resolved measurements Streak Camera Pump-Probe experiments • Ultrafast or fast pulses are used to excite (pump) and probe sample • Extremely fast carrier dynamics of samples can be measured Example setup 1 meter delay gives 3 nsec time delay Pump-Probe experiments X-Ray diffraction • X-rays have extreme small wavelengths compared to visible light (angstroms) • They can diffract off atomic planes! • X-Ray diffraction can be used to get information about crystals, nano and micro structure of materials etc. X-Rays • How are they produced? High energy electrons knock out core electrons XRD • Angle of diffraction • Width of peak gives information about crystal domain size XRD • Angle of diffraction tells lattice parameters • Width of peak gives information about crystal domain size • Molecular substances can be crystallized and analyzed by XRD