* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Summary of IPA in OS metastasis - Connective Tissue Oncology
Metabolic network modelling wikipedia , lookup
Transposable element wikipedia , lookup
Genomic imprinting wikipedia , lookup
Genomic library wikipedia , lookup
Human genome wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Gene therapy wikipedia , lookup
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
Copy-number variation wikipedia , lookup
Public health genomics wikipedia , lookup
Non-coding DNA wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Genetic engineering wikipedia , lookup
Point mutation wikipedia , lookup
Minimal genome wikipedia , lookup
Cancer epigenetics wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Gene expression programming wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Helitron (biology) wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Genome (book) wikipedia , lookup
Genome editing wikipedia , lookup
Genome evolution wikipedia , lookup
Gene expression profiling wikipedia , lookup
History of genetic engineering wikipedia , lookup
Microevolution wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Designer baby wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Oncogenomics wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
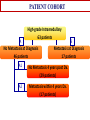
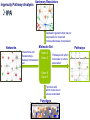
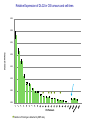
COMPREHENSIVE ANALYSIS OF DNA COPY NUMBER VARIATIONS AND GENE EXPRESSION IN OSTEOSARCOMA Nalan Gokgoz, Atta Goudarzi, Cheryl Wolting Jay S. Wunder and Irene L. Andrulis Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital Toronto, ON, Canada. Connective Tissue Oncology Society Meeting November 1, 2013 Identification and Characterization of Molecular Alterations in Osteosarcoma High resolution approaches to identify genes and pathways predictive of outcome in OS Gene expression profiling by Microarray Analysis Interrogation of biological pathways and networks Identification of the most relevant biological pathways for list of discriminative genes by Ingenuity Pathway Analysis Identification of the significant effectors and organizing networks in OS metastasis by Dynamo (Taylor and Chuang) Investigation of Copy Number Changes by Illumina SNP array technology Detection and characterization of alterations Analysis and visualization by Genome Studio and GAP Identification of significant recurrent targets by GISTIC PATIENT COHORT High-grade Intramedullary 63 patients A No Metastasis at Diagnosis 46 patients B Metastasis at Diagnosis 17 patients A1 No Metastasis 4 years post Dx. (29 patients) A2 Metastasis within 4 years Dx. (17 patients) MICROARRAY ANALYSIS Outcome of the Patients Presenting with “no Metastases” No Mets. Mets. within 4 yrs post Dx. 4 yrs post Dx. No Metastases 4 years post Dx (A1) vs Metastases within 4 years Dx (A2) 18981 cDNAs T-statistic p<0.001 (BrB Array Tools) n=53 genes for tumor classification/clustering Statistical validation by Leave-One Out cross-validation method Molecular validation by Real-Time Analysis Ingenuity Pathway Analysis Upstream Regulators Upstream regulators that may be responsible for observed increase/decrease in expression Networks Molecule Set Interactions and Relationships between molecules in set Gene A Gene C . . Gene X Gene Y Pathways with which molecules in set are associated Functions with which molecules in set are associated Functions Pathways Summary of IPA in OS metastasis Networks cell morphology, organization, hematopoiesis Pathways Rac/Rho, actin cytoskeleton Functions hematopoiesis, cell movement Regulators Fas, Fos, SP1, SREBF1 Signaling by Rho Family GTPases A1 – No mets A2 – Mets in 4 yrs Lower expression in A2 Higher expression in A2 GENETIC NETWORKS in OS METASTASIS Significant Networks • Transport • Translation • Signaling • • • The PRKCε, RASGPR3 and GNB2 networks differentially activated DLG2 network differentially organized The PRKCε, RASGPR3 and GNB2 networks are potential effectors of DLG2 Protein Kinase C Epsilon and Genetic Networks in Osteosarcoma Metastasis; A Goudarzi, N Gokgoz, M Gill, D Pinnaduwage, D. Merico, J.S Wunder and IL Andrulis, Cancer, 2013, 5, 372-403 Osteosarcoma and Copy Number Alterations Illumina 610-Quad Whole-genome genotyping beadchip Coverage includes >14,000 CNV regions and 550K evenly spaced TagSNPs from HapMap data High Resolution: Spacing 2.7 kb Includes markers in the unSPNable Genome Allows detection of SNPs, Copy Number Variation and Genotype Reference Genotype: Canonical genotype clusters (200 HapMap DNA genotype data) 44 Osteosarcoma Tumor DNA 25 of them with matched blood DNA Validation by Real Time PCR Complexity of OS Tumour Genome (Analysis by Genome Studio) Allele Frequency BB Blood DNA LogR Ratio Tumour DNA AB AA Genome Alteration Print (GAP) Analysis OS-2550_Chromosome 3 Recurrent Copy Number Gains in OS identified by GISTIC (Genome Identification of Significant Targets in Cancer) * * CDK4 MDM2 COL12A1 COL9A1 AF086303 q value * PPFIBP1 COL4A1 FGFR1OP2COL4A2 LIG4 MYR8 COPS3 NCORI PMP22 *Same family genes Recurrent Copy Number Losses in OS identified by GISTIC * q value * * *Same family genes 11q14.1 deletion in a matched tumor-blood DNA DLG2 Implication of DLG2 as a tumour suppressor in cancer One of the most disorganized genetic networks in metastatic OS tumours. The PRKCε, RASGPR3 and GNB2 networks are potential effectors of DLG2 Tumour suppressor function of dlg2 in Drosophila Scribble complex (SCRIB, DLG1-4 and LGL1/2) deregulation in Prostate Cancer DLG2 implicated in Wilms Tumour DLG2 (discs, large homolog 2) Channel associated protein of synapse 110 Chromosome 11q14 Member of the membrane-associated guanylate kinase (MAGUK) family. • PDZ domains; interaction with signalling proteins at postsynaptic sites • SH3 domains are found in proteins of signaling pathways regulating the cytoskeleton and regulate the activity state of adaptor proteins and other tyrosine kinases • GuKinase Domain; catalyzes ATP-dependent phosphorylation of GMP to GDP Gene: 2 MB, 33 alternative spliced transcripts Longest transcript :3.7KB, 26 Exons • Expression site: Brain, hypothalamus Relative Expression of DLG2 in OS tumours and cell-lines 0.350 0.300 DLG2/STAM2 0.250 0.200 0.150 0.100 0.050 0.000 OS Tumours Deletion of DLG2 gene detected by SNP array Work in Progress SiRNA Knockdown of the DLG2 Gene in U2OS 70% knockdown at 72 hours 140 120 100 80 60 40 20 0 48 72 96 The effect of DLG2 knockdown in Cell viability and growth by XTT assay Migration by scratch assay Sequencing of DLG2 gene for inactivating mutations CONCLUSION We identified a 53-gene expression signature that may predict outcome of OS patients with localized tumours. High-resolution approaches identified candidate pathways and networks that may be biologically relevant in OS. Cell morphology and organization pathways may be involved in OS metastasis. A large number of chromosomal aberrations were detected in OS tumours by SNP array technology. The DLG2 gene that is deleted in 20 percent of the OS cases and belonging to a significantly disorganized metastatic OS network and was chosen for further functional analysis. Further experiments will be performed to investigate the functional role of DLG2 in cell growth, proliferation and migration. Acknowledgement I. Andrulis S. Bull J. Wunder R. Parkes Andrew Seto Andrulis and Wunder Lab R. Kandel Mount Sinai Hospital Orthopedic Surgeons Hospital for Sick Children D.Malkin Vancouver General Hospital C.Beauchamp University of Washington E.Conrad III Royal Orthopedic Hospital R.Grimer Memorial Sloan-Kettering J.Healey Mayo Clinic M.Rock/ L.Wold During progression from tumour growth to metastasis, specific integrin signals enable cancer cells to detach from neighbouring cells, re-orientate their polarity during migration, and survive and proliferate in foreign microenvironments. There is increasing evidence that certain integrins associate with receptor tyrosine kinases (RTKs) to activate signalling pathways that are necessary for tumour invasion and metastasis. The effect of these integrins might be especially important in cancer cells that have activating mutations, or amplifications, of the genes that encode these RTKs.