* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Immunoglobulin and Monoclonal antibodies
Survey
Document related concepts
DNA vaccination wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Immune system wikipedia , lookup
Duffy antigen system wikipedia , lookup
Innate immune system wikipedia , lookup
Complement system wikipedia , lookup
Immunocontraception wikipedia , lookup
Autoimmune encephalitis wikipedia , lookup
Adaptive immune system wikipedia , lookup
Molecular mimicry wikipedia , lookup
Anti-nuclear antibody wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Transcript
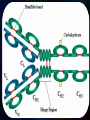
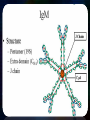
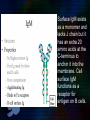
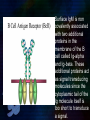
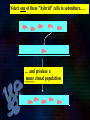
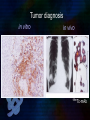
Immunoglobulin and Monoclonal antibodies Reported by Ganesh M.Sc.D endo student What is immunoglobulin ? Immunoglobulin's are glycoprotein molecules that are produced by plasma cells in response to an immunogen and which function as antibodies. The immunoglobulins derive their name from the finding that they migrate with globular proteins when antibody-containing serum is placed in an electrical field Immunoglobulin Fragments Structure/Function Relationships Fab Ag binding Valence = 1 Specificity determined by VH and VL Papain Fc ( crystallizable) Effector functions Fc Fab Immunoglobulin Fragments Structure/Function Relationships Ag Binding Complement Binding Site Binding to Fc Receptors Placental Transfer IgM Structure IgM normally exists as a pentamer (19S immunoglobulin) but it can also exist as a monomer. In the pentameric form all heavy chains are identical and all light chains are identical. Thus, the valence is theoretically 10. IgM has an extra domain on the mu chain (CH4) and it has another protein covalently bound via a S-S bond called the J chain. This chain functions in polymerization of the molecule into a pentamer. IgM Properties a) IgM is the third most common serum Ig. b) IgM is the first Ig to be made by the fetus and the first Ig to be made by a virgin B cells when it is stimulated by antigen. c) As a consequence of its pentameric structure, IgM is a good complement fixing Ig. Thus, IgM antibodies are very efficient in leading to the lysis of microorganisms. IgM Properties d) As a consequence of its structure, IgM is also a good agglutinating Ig . Thus, IgM antibodies are very good in clumping microorganisms for eventual elimination from the body. e) IgM binds to some cells via Fc receptors. f) B cell surface Ig Surface IgM exists as a monomer and lacks J chain but it has an extra 20 amino acids at the C-terminus to anchor it into the membrane. Cell surface IgM functions as a receptor for antigen on B cells. Surface IgM is non covalently associated with two additional proteins in the membrane of the B cell called Ig-alpha and Ig-beta. These additional proteins act as signal transducing molecules since the cytoplasmic tail of the Ig molecule itself is too short to transduce a signal. IgD IgD Properties a) IgD is found in low levels in serum; its role in serum uncertain. b) IgD is primarily found on B cell surfaces where it functions as a receptor for antigen. IgD on the surface of B cells has extra amino acids at C-terminal end for anchoring to the membrane. It also associates with the Ig-alpha and Ig-beta chains. c) IgD does not bind complement. IgE structure IgE Properties a) IgE is the least common serum Ig since it binds very tightly to Fc receptors on basophils and mast cells even before interacting with antigen. b) Involved in allergic reactions - As a consequence of its binding to basophils an mast cells, IgE is involved in allergic reactions. Binding of the allergen to the IgE on the cells results in the release of various pharmacological mediators that result in allergic symptoms. IgE Properties c) IgE also plays a role in parasitic helminth diseases. Since serum IgE levels rise in parasitic diseases, measuring IgE levels is helpful in diagnosing parasitic infections. Eosinophils have Fc receptors for IgE and binding of eosinophils to IgEcoated helminths results in killing of the parasite. d) IgE does not fix complement. clinical implications of human immunoglobulin classes IgM Increases (in adults) in: a) Waldenström's macroglobulinemia b) Trypanosomiasis c) Actinomycosis d) Carrión's disease (bartonellosis) e) Malaria f) Infectious mononucleosis g) Lupus erythematosus h) Rheumatoid arthritis I) Dysgammaglobulinemia (certain cases) Note: In the newborn, a level of IgM above 20 ng./dl is an indication of in utero stimulation of the immune system and stimulation by the rubella virus, the cytomegalovirus, syphilis, or toxoplasmosis. clinical implications of human immunoglobulin classes IgM Decreases in a) Agammaglobulinemia b) Lymphoproliferative disorders (certain cases) c) Lymphoid aplasia d) IgG and IgA myeloma e) Dysgammaglobulinemia f) Chronic lymphoblastic leukemia clinical implications of human immunoglobulin classes IgD Increases in a) Chronic infections b) IgD myelomas clinical implications of human immunoglobulin classes IgE Increases in a) Atopic skin diseases such as eczema b) Hay fever c) Asthma d) Anaphylactic shock e) IgE-myeloma clinical implications of human immunoglobulin classes IgE Decreases in a) Congenital agammaglobulinemia b) Hypogammaglobulinemia due to faulty metabolism or synthesis of immunoglobulins Monoclonal antibodies are antibodies that are identical because they were produced by one type of immune cell, all clones of a single parent cell Polyclonal antibodies are antibodies that are derived from different cell lines Epitope, also known as antigenic determinant, is the part of an antigen that is recognized by the immune system specifically by antibodies, B cells, or T cells. In 1975, Georges Kohler and Cesar Milstein first fused lymphocytes to produce a cell line which was both immortal and a producer of specific antibodies. The two scientists were awarded the Nobel Prize for Medicine in 1984 for the development of this "hybridoma." The value of hybridomas to the field was not truly appreciated until about 1987, when MAbs were regularly produced in rodents for diagnostics. Monoclonal antibodies Antibodies produced from a single clone of B cells. Produced by fusing a B cell secreting the desired antibody with a myeloma cell capable of growing indefinitely in tissue culture. Monoclonal antibodies all have identical antigen-binding sites. Thus they all bind to the same epitope with the same affinity. They are all of the same antibody class (isotype). Hybridomas Technique B lymphocytes can mutate into tumor cells that result in a type of cancer termed myeloma. - Myeloma cells become “immortal” and will grow indefinitely in culture. - Fusion of a single activated B cell and a myeloma cell will create a hybridoma that can grow indefinitely in culture Hybridoma Selection The “HAT Trick” Myeloma cells have been genetically engineered such that they can not use hypoxanthine, aminopterin, and thymidine (HAT medium) as a source for nucleic acid biosynthesis and will die in culture. Only B cells that have fused with the engineered myeloma cells will survive in culture when grown in HAT medium. Polyclonal antibodies Produced by: Many B cell clones Monoclonal Antibodies A single B cell clone Bind to: Multiple epitopes of all antigens used in the immunization A single epitope of a single antigen Antibody class: A mixture of different Ab classes (isotypes) All of a single Ab class Ag-binding sites: A mixture of Abs with different antigen-binding sites All Abs have the same antigen binding site Potential for cross-reactivity: High Low Monoclonal Antibodies Purified antigen molecule Inject antigen into mouse Kill the mouse and remove the spleen (containing antibody generating cells) spleen Each cell may have produced a different antibody spleen fuse dissociate + A Hybrid cell that can be cultured “immortalized” cell Select one of these "hybrid" cells to subculture…. … and produce a mono clonal population USES Measuring protein and drug levels in serum Typing tissue and blood Identifying infectious agents Identifying clusters of differentiation for the classification and follow-up therapy of leukemias and lymphomas USES Identifying tumor metastasis Identifying and quantifying hormones Immuno affinity Purification Used in several diagnostic tests to detect small amounts of drugs, toxins or hormones, e.g. monoclonal antibodies to HCG used in pregnancy test kits or diagnosis of AIDS by the ELISA test. USES monoclonal antibody can be coupled to another molecule like a fluorescent molecule to aid in imaging the target Or with a strongly-radioactive atom, such as Iodine-131 to aid in killing the target. Used in the radioimmuno detection and radioimmuno therapy of cancer, and some new methods can even target only cancerous cells. USES Monoclonal antibodies can be used to treat viral diseases, traditionally considered "untreatable". In fact, there is some evidence to suggest that antibodies may lead to a cure for AIDS. Monoclonal antibodies can be used to classify strains of a single pathogen, e.g. Neisseria gonorrhoeae can be typed using mAB. To identify and to trace specific cells or molecules in an organism. USES OKT3 (immunosuppressant ) an antibody to the T3 antigen of T cells, is used to alleviate the problem of organ rejection in patients who have had organ transplants. Affinity chromatography: 1. Bind antibody to a support matrix (e.g. sepharose gel) 2. Add protein mixture - antigen binds to antibody on support 3. Wash to remove unbound material 4. Lower pH - antibody releases the antigen - which is now free of contaminants Affinity chromatography antibody purification. Antigen can be bound to the support matrix in order to purify antigen-specific antibody from a polyclonal antiserum. From http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/A/Affi nityChrom.html Tumor diagnosis in vitro in vivo 99mTc-mAb Monoclonal antibodies for cancer treatment Three mechanisms that could be responsible for the cancer treatment. 1. mAbs act directly when binding to a cancer specific antigens and induce immunological response to cancer cells. Such as inducing cancer cell apoptosis, inhibiting growth, or interfering with a key function Monoclonal antibodies for cancer treatment 2. mAbs can be modified for delivery of a toxin, radioisotope, cytokine or other active conjugates. 3. it is also possible to design bispecific antibodies that can bind with their Fab regions both to target antigen and to a conjugate or effector cell Drawbacks of Monoclonal Antibodies Since monoclonal antibodies produce antibodies to a single determinant, they do not form the lattice necessary for precipitation and so cannot be used in precipitation assays, radial immunodiffusion, immunoelectrophoresis or agar gel diffusion. Very high titer antibodies also pose certain difficulties