* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download New Approaches to Chronic Anticoagulation
Neuropsychopharmacology wikipedia , lookup
Psychedelic therapy wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Pharmacognosy wikipedia , lookup
Prescription costs wikipedia , lookup
Drug design wikipedia , lookup
Metalloprotease inhibitor wikipedia , lookup
Neuropharmacology wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug discovery wikipedia , lookup
Drug interaction wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Theralizumab wikipedia , lookup
Dydrogesterone wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
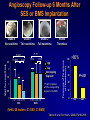
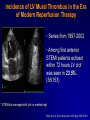
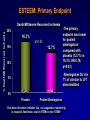
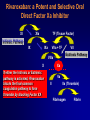
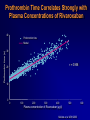
Welcome Ask The Experts March 24-27, 2007 New Orleans, LA New Approaches to Chronic Anticoagulation: Factor XA Inhibition C. Michael Gibson, MS, MD Associate Professor of Medicine Harvard Medical School Chief of Clinical Research Cardiology Division Beth Israel Deaconess Medical Center Boston, MA Meeting The Unmet Needs in Chronic Anticoagulation C. Michael Gibson, M.D., M.S. PCI Cure: Death / MI Remain High at One Year Despite PCI & Dual Antiplatelet Therapy Cumulative Hazard Rates Death / MI <48 hrs after rand 0.20 Denotes median Time to PCI PCI ≥ 48 hrs from rand and during initial hosp 0.20 PCI after hospital discharge 0.20 ASA ASA 0.15 0.15 0.10 0.10 0.05 0.05 ASA 0.15 0.10 ASA + Clopidogrel ASA + Clopidogrel 0.05 ASA + Clopidogrel RR:0.72 (0.51-1.01) RR:0.53 (0.27-1.06) 0.0 0.0 0 100 200 300 Days of Follow-up RR:0.70 (0.48-1.02) 0.0 0 100 200 300 Days of Follow-up 0 100 200 300 Days of Follow-up Lewis BS, et al. Am Heart J. 2005;150:1177-1184. Thrombus & Complex Lesion Remains One Month After STEMI % Thrombus on Angioscopy 100% 83% 80% 79% 70% 71% 60% 40% 20% • Angioscopic findings suggestive of plaque instability are extremely frequent (75% to 80% of the study population) as is the presence of clot even in the absence of clinical symptoms. • Only 16% of clot seen on angio 0% < 8 Days 8<& 10 < & > 15 Days (n=18) < 10 Days < 15 Days (n=14) (n=10) (n=14) Days after lysis or medical therapy Van Belle et al. Circulation. 1998;97:26-33. Angioscopy Follow-up 6 Months After SES or BMS Implantation Grade 1 Thin neointima P<.05 P=.63 P=.80 Stent Coverage Grade 2.5 2 1.5 Edge * * Body Overlapping Segment * 1 *P<.001 compared with the corresponding segment in the BMS. 0.5 0 Visible Thrombus P=.70 P<.001 P<.0005 Grade 2 Full neointima n=21 n=33 n=12 SES n=28 n=33 n=5 BMS Frequency of Persistence of Thrombus (%) Grade 0 No neointima 100 >80% 80 60 P=.031 40 20 0 SES BMS (N=46, 66 lesions: 33 SES, 33 BMS) Takano M, et al. Eur Heart J. 2006;27:2189-2195. Recurrent MI Following Lysis For STEMI 25% % Mortality 2 yrs 19.6% 20% p<0.0001 15% 10.1% 10% 5% n=19,265 n=836 0% No Reinfarction •Recurrent MI is associated with a doubling in mortality at 2 years •Patients at risk cannot be identified clinically Reinfarction Gibson CM et al, JACC 2003 Incidence of LV Mural Thrombus in the Era of Modern Reperfusion Therapy • Series from 1997-2002 • Among first anterior STEMI patients echoed within 72 hours LV clot was seen in 23.5%. (36/153) * STEMI pts managed with lytic or medical mgt Porter A et al. Coron Artery Dis. 2005 Aug;16(5):275-9 Meta Analysis of Anticoagulation Rates of Recurrent MI Rothberg et al. Ann. Int. Med. 2005;143:241-250 ASPECT II: Coumadin is Efficacious in ACS, But Discontinuation is Common 999 Pts within 8 wks of UA or Acute MI Rx : ASA 80 mg; Coumadin (INR 3-4); or Combination: ( INR 2-2.5)+ ASA 80 mg Efficacy Safety % Death,MI,CVA 30 Major Bleed Tranfusion Minor Bleed 20 15 8 10 5 1 1 1 1 2 1 0 ASA Rate of Discontinuation 10% Coumadin Combo 19% 20% van Es et al Lancet 360:109,2002 OASIS 2: Impact of Anticoagulation Discontinuation P=0.02 P=0.33 P=0.005 P=0.16 21.3 20 18.5 Std Rx 16.5 % Pts Oral A/C + ASA 15 11.9 10 8.9 9 7.8 6.1 5 0 Compliance: Good (% on Oral AC) >70 % Bad Good Bad < 70% > 70% < 70% CVD,MI,CVA CVD, MI, CVA, Rehosp UA OASIS Inv JACC 37:475,2001 Anticoagulation Therapy Agent Disadvantages Heparin • • • Parenteral administration Risk of heparin-induced thrombocytopenia (HIT) Narrow therapeutic window (low bioavailability, short halflife) Warfarin • Requires frequent monitoring due to: – Narrow therapeutic window – Unpredictable pharmacology – Multiple drug–drug and food–drug interactions – Increased risk of major and minor bleeds LMWH • • Parenteral administration Risk of heparin-induced thrombocytopenia (HIT) Indirect Xa Inhibitor (e.g. fondaparinux) • • • Parenteral administration Long half-life Limitations related to special patient populations Direct Thrombin Inhibitors • • Parenteral administration Current applications limited to cardiovascular management Albans S et al. Eur J Clin Invest 2005;35(Suppl 1):12-20. Nadia Comaneci 1976: First Perfect Score of 10 In Olympics Anticoagulation is like a balance beam performance Both efficacy and safety are important, and if you fail to balance efficacy and safety, the patient may get hurt The Search for an Anticoagulant That Balances Safety and Efficacy Thrombosis Optimal Safety and Efficacy Bleeding Dose (concentration) of Anticoagulant Meeting the Unmet Need in Long Term Anticoagulation in ACS •Unmet anticoagulant needs: • Safe • Effective • Ease of use • One dose • No monitoring • Unaffected by diet ESTEEM: Primary Endpoint % Death/MI/Recurrent Ischemia Death/MI/Severe Recurrent Ischemia 20% 16.3% p=0.03 15% 12.7% 10% •The primary endpoint was lower for pooled ximelagatran compared with placebo (12.7% vs 16.3%, HR 0.76, p=0.03) •Ximelagatran Dc’d in 7% of pts due to LFT abnormalities 5% n=1,245 n=638 0% Placebo Pooled Ximelagatran • Oral direct thrombin inhibitor (IIa), no coagulation monitoring is required, fixed dose, eval in STEMI or non-STEMI Rivaroxaban: An Oral Direct Xa Inhibitor • Direct, specific, competitive Factor Xa inhibitor • Inhibits free and fibrin-bound Factor Xa activity, and prothrombinase activity • Does not directly inhibit thrombin, but inhibits thrombin generation via inhibition of Factor Xa activity • Does not affect agonist-induced platelet aggregation, and therefore has no direct effect on primary hemostasis • Does not require a cofactor • No interaction with aspirin, enoxaparin, digoxin, naproxen, ranitidine, or antacids Perzborn et al., J Thromb Haemost 2005; ICT 2004; Depasse et al., ISTH 2005; Kubitza et al., J Clin Pharmacol 200; ASH 2005; Fareed et al., ISTH 2005 Rivaroxaban: a Potent and Selective Oral Direct Factor Xa Inhibitor TF (Tissue Factor) XIa XI Intrinsic Pathway IX IXa VIIa + TF VIIIa X If either the Intrinsic or Extrinsic pathway is activated, Rivaroxaban blocks the final common coagulation pathway to form thrombin by blocking Factor XA VII Extrinsic Pathway Xa Va II IIa (Thrombin) Fibrinogen Fibrin Prothrombin Time Correlates Strongly with Plasma Concentrations of Rivaroxaban 40 Prothrombin time (s) Prothrombin time Model 30 r = 0.958 20 10 0 0 100 200 300 400 500 Plasma concentration of Rivaroxaban (µg/l) Kubitza et al. ASH 2003 600 Rivaroxaban: Persistent Antithrombotic Effect Out to 24 Hours % Inhibition of Factor Xa 70 Rivaroxaban 1.25 mg (n=8) Rivaroxaban 5 mg (n=6) Rivaroxaban 10 mg (n=8) Rivaroxaban 20 mg (n=7) Rivaroxaban 40 mg (n=8) Rivaroxaban 80 mg (n=6) Placebo (n=25) 60 Anti-Xa Activity 50 40 30 20 10 0 -10 0 2 4 6 8 10 12 14 16 18 20 Time (hours) ► All once-daily dosage regimens demonstrated Xa inhibition for out to 24 hours ► These results provided foundation for selection of once-daily dosing regimen for Phase III programs Kubitza, et al. Clin Pharmacol Ther 2005;78(4):412-21. 22 24 Rivaroxaban: Human Pharmacokinetics • Dose peaks in 2.5–4 hours, half life 5-9 hours (11-13 hours in elderly) • One dose will be selected for clinical use • No monitoring required given consistent dose response • Dual modes of excretion •Renal (66%), but no excess bleeding associated with CrCl to date •Faecal/biliary (28%) • Minimal drug/drug interactions, no major circulating metabolites, no drug accumulation Kubitza et al., Eur J Clin Pharmacol 2005; Eriksson et al., J Thromb Haemost 2006; Turpie et al., J Thromb Haemost 2005; Kubitza et al., ISTH 2005; Kubitza et al., ASH 2005; Kubitza et al., J Clin Pharmacol 2006 Rivaroxaban: Anti-Thrombotic Efficacy Thrombus reduction (%) 100 *P<0.05 ; **P<0.01 ** 80 * 60 • Arterial thrombosis rabbit arteriovenous shunt model • Rivaroxaban dosedependently prevented arterial thrombosis 40 20 0 0.3 1.0 3.0 Rivaroxaban (mg/kg) p.o. Rivaroxaban Safety: Bleeding Time Tail Transection Bleeding Time in Rats Compound X-fold prolongation of bleeding time at ED50 (control =1) Rivaroxaban [po] 1.8 Enoxaparin [sc] 2.2 Ximelagatran [po] 3.7 Dabigatran [po] 4.9 Warfarin [po] > 6.3 Bleeding time comparable to enoxaparin Lower compared to thrombin inhibitors or warfarin Rivaroxaban: Bleeding Time with Combination Therapy Tail Transection Bleeding Time in Rats Compounds Clopidogrel 1 mg/kg [po] Aspirin 3 mg/kg [po] Clopidogrel 1 mg/kg [po] Aspirin 3 mg/kg [po] + Rivaroxaban 0.1 mg/kg [iv] X-fold prolongation of bleeding time 2.1 +/- 0.3 2.5 +/- 1 Similiar Bleeding Times Dose–response Relationship: Safety And Efficacy DVT, PE, and all-cause mortality Major, post-operative bleeding Incidence rate % 30 20 10 0 0 5 10 15 20 25 30 35 40 Enoxaparin 40 mg Total daily dose (mg) of Rivaroxaban Eriksson et al., Circulation 2006 Safety and Tolerability • Rivaroxaban was well tolerated, with similar incidence of AEs as enoxaparin • Rivaroxaban did not affect ECG parameters • Rivaroxaban did not have any substance-specific effects on laboratory parameters (except for clotting tests) • LFT increases with BAY 597939 did not exceed the level observed with enoxaparin – There was no dose-dependent increase in transaminase levels Liver function test (LFT) ALT > 3× ULN % Rivaroxaban Enox 5 mg 10 mg 20 mg 30 mg 40 mg 40 mg 5/119 4.2 6/133 4.5 4/133 3.0 7*/129 5.4 5/127 3.9 10/140 7.1 *One patient had ALT >3× ULN and bilirubin >2× ULN (occurring before first intake of study drug) Safety Rivaroxaban Efficacy Ease CM Gibson 2007 Rivaroxaban: Conclusions • Is a selective, reversible, active-site directed Factor Xa inhibitor that inhibits coagulation triggered by both the collagen (intrinsic) and tissue factor (extrinsic) pathways • Reduces thrombus formation in both venous and arterial thrombosis models • Has a bleeding risk comparable to Enoxaparin, and lower compared to thrombin inhibitors and Warfarin, in preclinical in vivo models CM Gibson 2007 Rivaroxaban: Conclusions • Reaches Peak (Cmax)) in 2.5–4 hours; half-life of 5–9 hours at steady state (little longer in older) • Dual modes of excretion: Renal (66%) & Faecal / biliary (28%) • No substantial accumulation after multiple dosing, few drug interactions • Dose dependent prolongation of prothrombin time CM Gibson 2007 Rivaroxaban Clinical Development Program • Ongoing evaluation in acute and chronic settings for prevention and treatment of multiple venous and arterial indications Target Enrollment Phase II-III 35,000 - 40,000 CM Gibson 2007 Question & Answer Thank You! Please make sure to hand in your evaluation and pick up a ClinicalTrialResults.org flash drive