* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Document
History of invasive and interventional cardiology wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Baker Heart and Diabetes Institute wikipedia , lookup
Rheumatic fever wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Artificial heart valve wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Heart failure wikipedia , lookup
Coronary artery disease wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Aortic stenosis wikipedia , lookup
Electrocardiography wikipedia , lookup
Cardiac surgery wikipedia , lookup
Jatene procedure wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Myocardial infarction wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Heart arrhythmia wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
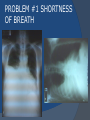
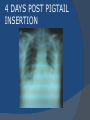
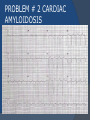
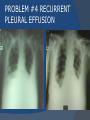
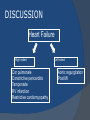
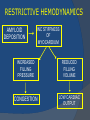
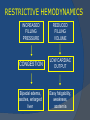
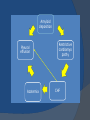
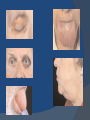
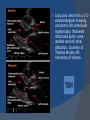
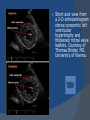
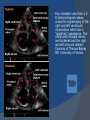
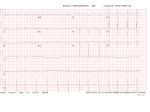
July 5, 2007 Anne Marie Kathryn P. Ingente MD LEARNING OBJECTIVES To present a case of CHF secondary to restrictive cardiomyopathy secondary to cardiac amyloidosis To discuss the diagnosis and management of cardiac amyloidosis IDENTIFYING DATA 65-year-old Filipino male, married, resident from US (+) HPN (since 1991) (+) DM 2 (since 1991) CHIEF COMPLAINT Difficulty of breathing HISTORY January 2006: (+) easy fatigability December 2006: (+) (+) (+) (+) easy fatigability 2-pillow orthopnea bipedal edema occasional cough w/ whitish phlegm (-) fever; (-) chest pain; (-) palpitations admitted at Stanford University Medical Center (Palo Alto Medical Foundation) 2D-ECHO (Palo Alto Medical Clinic; Dec 28, 2006) Concentric LVH. Small left ventricular cavity. Mildmoderate LV systolic dysfunction (EF 40-50%). Normal RV size. RV hypertrophy. Moderate RV systolic dysfunction. Right and left atrial sizes are within normal limits. Mild thickening of the aortic and pulmonic valves. Large right pleural effusion. Ascites and small pericardial effusion noted. HISTORY February 2007: (+) (+) (+) (+) admitted at Stanford responded to diuretics, salt and fluid restriction easy fatigability shortness of breath bipedal edema occasional cough, with scanty whitish phlegm HISTORY MAY 1, 2007; Stanford University Medical Center Right heart catheterization with right ventricular biopsy RV biopsy was remarkable for CARDIAC AMYLOIDOSIS. Immunofixation Electrophoresis of Serum: Elevated free lambda light chains HISTORY MAY 16, 2007; Stanford University Medical Center Bone marrow biopsy with flow cytometric immunophenotyping was done. HISTORY BONE MARROW BIOPSY: - Moderate monoclonal plasmacytosis (10-20%) consistent with a plasma cell dyscrasia FLOW CYTOMETRIC IMMUNOPHENOTYPING: - Lambda light chain-restricted plasma cells HISTORY May 31, 2007; Makati Medical Center - sought consult for continuation of treatment - easy fatigability, shortness of breath, bipedal edema, orthopnea REVIEW OF SYSTEMS Skin: (+) periorbital bruising, (-) urticaria, (-) rash Bones, joints, muscles: (-) pain, (-) muscle weakness Hematopoietic: (-) bleeding; (-) delayed clotting HEENT: (-) headache, (-) blurring of vision, (-) tinnitus, (-) hearing loss, (+) dysphagia, (+) hoarseness REVIEW OF SYSTEMS ABDOMEN: (-) pain, (-) bloatedness, (+)constipation (-) diarrhea GENITOURINARY: (-) hesitancy, intermittency, frequency (-) hematuria (-) dysuria EXTREMITIES: (+) pricking sensation on the R tibia, (+) numbness on tips of toes and fingers PAST MEDICAL HISTORY (+) S/P Appendectomy – 1960s (+) S/P Surgery for Carpal Tunnel Syndrome – 1991 (+) Gout – 1980 (Allopurinol 100mg OD) (+) Dyslipidemia – 1990s (Simvastatin 20mg OD) No asthma, no allergies, no history of TB No prior MI or CVA PAST MEDICAL HISTORY Maintenance meds: Glipizide 5mg OD Insulin Simvastatin 20mg OD Allopurinol 100mg OD Hydrocholorothiazide 25mg OD Bumetanide 1mg/tab 2 tabs BID (4mg/day) KCl 10 mEq tab 1 tab with each tablet of Bumetanide, up to 4 tabs daily FAMILY MEDICAL HISTORY (+) HPN – mother (+) DM – mother (+) heart disease – father (-) asthma (-) cancer PERSONAL & SOCIAL HISTORY Non-smoker Occasionally drinks Retired architect PHYSICAL EXAMINATION BP 110/70 afebrile HR 104 reg RR 22 Conscious, coherent, conversant Pink palp conjunctivae, anicteric sclerae, (+) periorbital discoloration Trachea midline, thyroid not palpable, no CLAD, JVP 12 cm H20, no carotid bruit PHYSICAL EXAMINATION Lungs: symmetric chest expansion, no retractions, dullness to percussion on the R mid basal lung field, decreased breath sounds on the R mid to base, fine crackles on the left base Heart: adynamic precordium, outer border 2 fingers outside the LMCL, tachycardic, regular rhythm,distinct heart sounds, no murmurs PHYSICAL EXAMINATION Protuberant abdomen with bulging flanks, normoactive bowel sounds, liver and spleen palpable, liver edge felt at 5 cm below the right costal margin, (+) dullness at Traube’s space, (+) shifting dullness. (+) Grade 3 bipedal pitting edema, dorsalis pedis strong and equal, pink nail beds SALIENT FEATURES 65-yr-old Filipino male Diagnosed with cardiac amyloidosis Came for continuation of treatment Persistent shortness of breath, easy fatigability, bipedal edema, orthopnea Periorbital edema Dullness on percussion on the R mid to basal lung field, decreased breathsounds on R mid to base, fine crackles L base Outer border 2 fingers outside the LMCL, tachycardic, regular rhythm, no murmurs Protuberant abdomen with bulging flanks, NABS, liver and spleen palpable, liver edge felt at 5cm below the R costal margin (+) dullness at Traube’s space, (+) shifting dullness Grade 3 pitting bipedal edema ADMITTING DIAGNOSIS Congestive Heart Failure secondary to Restrictive Cardiomyopathy secondary to Cardiac Amyloidosis Hypertensive atherosclerotic disease Diabetes Mellitus Gout PROBLEM #1 SHORTNESS OF BREATH Chest USG Result showed massive amount of anechoic free fluid in the right hemithorax with a volume of at least 1100cc. PLEURAL FLUID Protein 2.7 gm% Glucose 204 mg% LDH 57 U/L RBC 584 WBC 3 Segmenters 3 20cc yellow, hazy; specimen with clot No microorganisms seen; WBC 4-6/OIF No growth in 5 days 4 DAYS POST PIGTAIL INSERTION PROBLEM # 2 CARDIAC AMYLOIDOSIS 2D- ECHO concentric LVH with global hypokinesia. Ejection fraction of 39% by simpson and 45% by teicholz. Dilated left atrium without evidence of thrombus. Normal right atrial and right ventriuclar dimensions. Normal main pulmonary artery, aortic root and proximal ascending aortic dimensions. Calcified right coronary, non coronary and left coronary cusps of the aortic valve with normal valve mobility. Pericardial effusion mild to moderate. Normal tricuspid valve and pulmonic valve. Color flow and Doppler study showed mitral regurgitation, mild. Aortic regurgitation, trivial. Tricuspid regurgitation mild. Pulmonic regurgitation, mild. Mild pulmonary hypertension. Restricted filling pattern of mitral valve leaflet velocity flow. PROBLEM # 3 INCREASED CREATININE 6/2 Na 134 K 3.5 BUN 61 Crea 2.1 Mg Ca 6/3 3.8 2.2 6/5 6/7 127 135 3.9 3.9 58 59 2.0 1.8 6/8 3.7 6/13 6/15 6/17 134 133 132 134 4.3 4.5 4.4 4.1 43 1.8 1.7 9.9 6/9 1.6 1.6 1.9 PROBLEM #4 RECURRENT PLEURAL EFFUSION FINAL DIAGNOSIS Congestive Heart Failure secondary to Restrictive cardiomyopathy secondary to cardiac amyloidosis Hypertensive atherosclerotic cardiovascular disease Pleural effusion secondary to CHF Chronic renal insufficiency secondary to cardiac decompensation Diabetes Mellitus Gout DISCUSSION Heart Failure Right-sided Cor pulmonale Constrictive pericarditis Tamponade RV infarction Restrictive cardiomyopathy Left-sided Aortic regurgitation Post MI RESTRICTIVE CARDIOMYOPATHY Defined as heart- muscle disease results in impaired ventricular filling with normal or decreased diastolic volume of either or both ventricles Usually results from increased stiffness of the myocardium Causes pressure within the ventricles to rise precipitously with only small increase in volume Affects either or both ventricles May cause symptoms and signs of R or L ventricular failure Often R sided findings predominate Considered in a patient presenting with heart failure but no evidence of cardiomegaly or systolic dysfunction RESTRICTIVE HEMODYNAMICS AMYLOID DEPOSITION INC STIFFNESS OF MYOCARDIUM INCREASED FILLING PRESSURE REDUCED FILLING VOLUME CONGESTION LOW CARDIAC OUTPUT RESTRICTIVE HEMODYNAMICS INCREASED FILLING PRESSURE REDUCED FILLING VOLUME CONGESTION LOW CARDIAC OUTPUT Bipedal edema, ascites, enlarged liver Easy fatigability, weakness, azotemia WHAT DOES AMYLOID DO TO THE HEART? Amyloid deposition can disturb the tissue architecture and lead to organ dysfunction J Clin Pathol 2005; 58: 125133 WHAT HAPPENED TO OUR PATIENT? Amyloid deposition Pleural effusion Azotemia Restrictive cardiomyo pathy CHF WHAT IS AMYLOID? Nonbranching fibrillar structure, an indefinite length and a 9.5 nm width Organized into a pure beta pleated sheet configuration making it highly insoluble Formation is not clearly understood But is thought that development of amyloid is a result of cleavage of the light chains of the immunoglobulins followed by aggregation of these light chains into beta pleated sheet WHAT IS AMYLOIDOSIS Refers to the deposition of amyloid protein in organs and tissues Protein fragments of normal antibody molecules produced by plasma cells in the bone marrow Amyloid is deposited in various organs and tissues including tongue, intestines, skeletal and smooth muscles, nerves, skin, ligaments, heart, liver, spleen and kidneys AMYLOID DEPOSITS STAIN AS RED WITH CONGO RED STAIN AND SHOW APPLEGREEN BIREFRINGENCE UNDER POLARIZED LIGHT INCIDENCE 8 cases per million per year Occurs in both sexes 2:1 (males:females) Peak occurrence at 60-67 y.o ○ Mayo Reference services publications Sept 2002 SYMPTOMS SYNDROMES Infiltrative cardiomyopathy with restrictive hemodynamics Nephrotic range proteinuria w/ or without renal insufficiency Indiopathic peripheral neuropathy Unexplained hepatomegaly Unexplained splenomegaly Carpal tunnel syndrome Macroglossia Gastrointestinal symptoms AMYLOID TYPES TYPE PRIMARY (AL) FIBRIL COMPOSITION Monoclonal light chain TREATMENT Chemotherapy; Stem cell transplant FAMILIAL (ATTR) Mutated transthyretin Liver transplant SENILE (ATTR) Normal transthyretin Supportive SECONDARY (AA) Protein A Control of inflammation Hemodialysisassociated Beta-2microglobulin Supportive CARDIAC AMYLOIDOSIS MYOCARDIUM AMYLOID CARDIAC CONDUCTION SYSTEM PERIVASCULAR VALVES (SMALL INTRAMURAL VESSELS) PE in Patients with Cardiac Amyloidosis Elevation of jugular venous pressure Hypotension may be caused by a low cardiac output Orthostatic hypotension R sided 3rd heart sound is occasionally heard Murmur of tricuspid or mitral regurgitation is occasionally heard ECG FINDINGS Low voltage in the limb leads occurring in approximately 50% Conduction abnormalities Atrial fibrillation Pseudoinfarct patterns ECHOCARDIOGRAPHY noninvasive test of choice Left ventricular wall thickening with evidence of diastolic dysfunction is the earliest echocardiographic abnormality In more advanced disease, wall thickening progresses resulting in cardiomyopathy with a nondialted or small LV cavity Biatrial enlargement occurs, and the R ventricle may dilate Mitral and aortic valves may become thickened Doppler evaluation of transmitral, blood flow velocity shows a restrictive filling pattern Amyloid infiltration of the heart results in increased echogenicity Described as “granular, sparkling” appearance of the myocardium and it resulted in unusually high quality myocardial visualization “sparkling pattern is not sensitive because only a minority 26% has it Long axis view from a 2-D echocardiogram showing concentric left ventricular hypertrophy, thickened mitral and aortic valve leaflets and left atrial dilatation. Courtesy of Thomas Binder, MD. University of Vienna. Short axis view from a 2-D echocardiogram shows concentric left ventricular hypertrophy and thickened mitral valve leaflets. Courtesy of Thomas Binder, MD. University of Vienna. Four chamber view from a 2D echocardiogram shows concentric hypertrophy of the right and left ventricular myocardium which has a "sparkling" appearance. The mitral and tricuspid valves are thickened and the right and left atria are dilated. Courtesy of Thomas Binder, MD. University of Vienna VOLTAGE TO MASS RATIO Left ventricular thickening due to amyloid infiltration may be d misdiagnosed on echo as LEVH. However, unlike true LVH, left ventricular thickening in cardiac amyloidosis is associated with a decrease in ECG voltage DIAGNOSIS Presence of cardiac amyloidosis should be ruled out in any patients with unexplained heart failure TISSUE BIOPSY Demonstrating amyloid deposits on endomyocardial biopsy Amyloid deposits on histologic examination of a biopsy from other tissues (abdominal fat pad, rectum or kidney) Monoclonal paraprotein Serum or urine monoclonal paraprotein NUCLEAR IMAGING Increased cardiac uptkae of radiolabeled Tc in patient with amyloid heart disease Not sensitive CARDIOVASCULAR MAGNETIC RESONANCE Global and subendocardial late enhancement of the myocardium Sensitivity of this test was not assessed and the predictive value of this test remains undetermined BNP and N-terminal proBNP Increased in heart failure Seen in patients with AL amyloidosis before the onset of clinical heart failure and are a marker of cardiac involvment. Sensitivity 93% Specificity of 90% TREATMENT Usually ineffective and generally consists of supportive measures TREATMENT OPTIONS Melphalan + stem cell transplantation Melphalan + dexamethasone High-dose dexamethasone Thalidomide + dexamethasone SWISS MED WKLY 2006; 136: 715-720 SUPPORTIVE TREATMENT Salt restriction Judicious diuretic use Control of neuropathic pain Transplantation of organs Prognosis of AL amyloidosis Median survival 6-9 months in those with heart failure 1.1 years in those with any sign of cardiac involvement