* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ch 12- 13 - Phillips Scientific Methods
Survey
Document related concepts
Cracking (chemistry) wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Asymmetric induction wikipedia , lookup
Hydrogenation wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Homoaromaticity wikipedia , lookup
George S. Hammond wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Ene reaction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Stille reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Aromaticity wikipedia , lookup
Hydroformylation wikipedia , lookup
Transcript
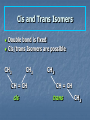
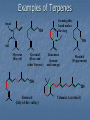
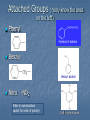
Alkenes, Alkynes, and Aromatic Compounds (Chapter 12 and 13) Alkenes and Alkynes Unsaturated contain carbon-carbon double and triple bond to which more hydrogen atoms can be added. Alkenes: carbon-carbon double bonds Alkynes: carbon-carbon triple bonds. Naming Alkenes and Alkynes IUPAC nomenclature rules for alkenes and alkynes are similar to alkanes. Step 1. Name the parent compound. Find the longest chain containing the double or triple bond, and name the parent compound by adding the suffix –ene or –yne to the name of the main chain. Step 2: Number the carbon atoms in the parent chain, beginning at the end nearest to the double or triple bond. If the multiple bond is an equal distance from both ends, begin numbering at the end nearer the first branch point. The number indicates which carbon the multiple bond is AFTER. (i.e. between 2 and 3 is 2-) Step 3: Assign numbers and names to the branching substituents, and list the substituents alphabetically. Use commas to separate numbers, and hyphens to separate words from numbers. Step 4. Indicate the position of the multiple-bond carbon. If more than one multiple bond is present, identify the position of each multiple bond and use the appropriate ending diene, triene, tetraene, and so forth. Step 5. Assemble the name. Naming Alkenes and Alkynes When the carbon chain has 4 or more C atoms, number the chain to give the lowest number to the double or triple bond. 1 2 3 4 CH2=CHCH2CH3 1-butene but-1-ene CH3CH=CHCH3 2-butene but-2-ene 2-butyne but-2-yne CH3C CCH3 Assigning Priority Alkenes and alkynes are considered to have equal priority In a molecule with both a double and a triple bond, whichever is closer to the end of the chain determines the direction of numbering. In the case where each would have the same position number, the double bond takes the lower number. In the name, “ene” comes before “yne” because of alphabetization. Learning Check Write the IUPAC name for each of the following unsaturated compounds: A. CH3CH2CCCH3 CH3 CH3 B. CH3C=CHCH3 C. Answers: 2-pentyne 2-methyl-2-butene 3-methylcyclopentene (doesn’t need a “1” before cyclopentene, because the double bond must be in position 1) Dienes, Trienes, Polyenes Alkenes with more than one double bond are named as alkadienes, alkatrienes, etc. Compounds with several double bonds are referred to more generally as polyenes (Greek: poly, many) CH2 =CHCH2 CH=CH2 1,4-Pentadien e CH3 CH2 =CCH=CH2 2-Meth yl-1,3-b utadiene (Isoprene) 1,3-Cyclopentad iene Cis-Trans Isomerism Methane is tetrahedral, ethylene is planar, and acetylene is linear as predicted by the VSEPR theory discussed earlier. The two carbons and the four atoms that make up the double bond in alkenes lie in a plane. Unlike carbon-carbon single bonds, rotation around carbon-carbon double bond is not possible. As a result, a new kind of isomerism is possible for alkenes. Because rotation is not possible around carbon-carbon double bonds, there are two different kinds of 2-butenes. These are known as cis-trans isomers. Pick up a kit. butene Build cis-2-butene and trans-2Chapter One 12 Cis and Trans Isomers Double bond is fixed Cis/trans Isomers are possible CH3 CH3 CH = CH cis CH3 CH = CH trans CH3 Cis- and Trans- terminology If alkenes have two different substituents at each end of the C=C then they can exist as stereoisomers because there is restricted rotation of the double bond. For example: all terminal alkenes (begin or end with a C=CH2) do not exist as cis- and trans- isomers. all 1,1-symmetrically disubstituted alkenes (has a C=CR2 unit) do not exist as cis- and trans-. alkenes with the R-CH=CH-R unit can exist as cis- and trans- isomers. In cis isomers, two methyl groups are close together on the same side of the double bond. In trans isomer, two methyl groups are far apart on opposite side of the double bond. Both cis and trans isomers have the same formula and connections between the atoms but have different three dimensional structures because the way the groups are attached to the carbons. Cis-trans isomerism occurs in an alkene whenever each double bond carbon is bonded to two different substituent groups. Cis-trans isomerism is not possible if one of the double bond carbons is attached to two identical groups. Cis / Trans Another way of “viewing” this: The orientation of the Carbon atoms of the parent chain determines whether an alkene is cis or trans. Which is cis? trans? Cis/trans in alkadienes, trienes See exp. 12.4. To determine the # of stereoisomers, use the formula 2n Applying Organic to Biochemistry: Unsaturated Fatty Acids Fatty acids in vegetable oils are omega-6 acids (the first double bond occurs at carbon 6 counting from the methyl group) A common omega-6 acid is linoleic acid CH3CH2CH2CH2CH2CH=CHCH2CH=CH(CH2)7COOH 6 linoleic acid, a fatty acid Trans Fats In vegetable oils, the unsaturated fats usually contain cis double bonds. During hydrogenation, some cis double bonds are converted to trans double bonds (more linear) causing a change in the fatty acid structure The more linear the chains, the closer together and more “solid”. “Clogs” arteries Triglycerides Glycerol + 3 fatty acids = Triglyceride We will do the reaction for how they are formed in next unit (esterfication) If an unsaturated fatty acid has multiple bonds (polyunsaturated), enzymes in the body can attach polar proteins and as a result create HDL (high density lipoprotein). Much better than LDL! (which clogs arteries) Polyunsaturated (best fat) >monounsaturated > saturated Trans Fats In the US, it is estimated that 2-4% of our total Calories is in the form of trans fatty acid. trans fatty acids behave like saturated fatty acids in the body. Many studies report that trans fatty acids raise LDL-cholesterol. Some studies also report that trans fatty acid lower HDLcholesterol Fats and Atheroschlerosis Inuit people of Alaska have a high fat diet and high blood cholesterol levels, but a very low occurrence of atherosclerosis and heart attacks. Fat in the Intuit diet was primarily from fish such as salmon, tuna and herring rather than from land animals (as in the American diet). Omega-3 Fatty Acids Fatty acids in the fish oils are mostly the omega-3 type (first double bond occurs at the third carbon counting from the methyl group). linolenic acid 18 carbon atoms CH3CH2CH=CHCH2CH=CHCH2CH=CH(CH2)7COOH eicosapentaenoic acid (EPA) 20 carbon atoms CH3CH2(CH=CHCH2)5(CH2)2COOH Atherosclerosis Plaques of cholesterol adhere to the walls of the blood vessels Blood pressure rises as blood squeezes through smaller blood vessels Blood clots may form Omega-3 fatty acids decrease the “sticking” of blood platelets (fewer blood clots) Properties of Alkenes and Alkynes Nonpolar, insoluble in water, soluble in nonpolar organic solvents. Less dense than water as a result floats on water. London (van der Waals) forces between molecules (weak) Flammable Nontoxic Alkenes display cis-trans isomerism whereas alkynes do not. Both alkenes and alkynes are chemically reactive. 27 Terpenes (useful for Herbfest) Terpene: a compound whose carbon skeleton can be divided into five-carbon units identical with the carbon skeleton of isoprene CH3 CH2 =C-CH=CH2 2-Methyl-1,3-b utadien e (Is op ren e) head tail C 1 2 3 4 C-C-C-C Isoprene u nit Example of an important principle of the molecular logic of living systems Small subunits are combined (and modified) to make larger molecules (polymerization, discussed later) In nature, reactions carried out by enzymes (catalysts) Examples of Terpenes head OH formin g th is bond makes the ring OH tail Myrcene (Bay oil) Geraniol (Ros e and other flow ers) Limonen e (Lemon an d oran ge) Menth ol (Peppermint) OH OH Farnes ol (Lily-of-th e valley) Vitamin A (retinol) Kinds of Organic Reactions Addition reactions: A substance add to the multiple bond of an unsaturated reactant to yield a saturated product that has only single bonds. 4 types- hydrogenation, halogenation, reaction with acids, hydration. See p. 363-370 Elimination reaction: In which a saturated reactant yields an unsaturated product by losing groups from two adjacent carbons. We will do these in the next unit (alcohols to alkenes). Not on this test Chapter One 30 Substitution reaction: In which an atom or group of atoms in a molecule is replaced by another atom or group of atoms. Ex- alkanes or aromatics (benzene) Rearrangement reaction: In which a molecule undergoes bond reorganization to yield an isomer. Not on this test Oxidation /Reduction: See next slide Chapter One 31 Oxidation/Reduction Oxidation-The loss of electrons (LEO or OIL) and /or hydrogens by a compound. (Can also be the gain of oxygen) Reduction- The gain of e- (GER or RIG) and or H’s by a compound. (Can also be the loss of O) The more saturated a hydrocarbon is, the more reduced it is. Alkanes are the most reduced. Alkenes are more oxidized than alkanes (less H bonds) and alkynes are more oxidized than alkenes. 32 Redox Continued Alkanes can become oxidized under strong heat and a catalyst. This is what happens in alkane “cracking”. Alkanes (higher molecular wt)Alkenes (lower molecular wt) **Remember….alkanes are more reduced; alkenes are more oxidized (alkynes even more oxidized) Chapter One 33 Reactions require Catalysts Are used to initiate and lower activation energy of reaction, but not used up in the rxn. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process regenerating the catalyst. The following is a typical reaction scheme, where C represents the catalyst, X and Y are reactants, and Z is the product of the reaction of X and Y: X + C → XC (1); Y + XC → XYC (2); XYC → CZ (3); CZ → C + Z (4) Although the catalyst is consumed by reaction 1, it is subsequently produced by reaction 4, so for the overall reaction: X + Y → Z Chapter One 34 Catalysts con’t For reaction X+Y Z, where a catalyst is used: X + C → XC (1) (catalyst is “alone” at start) Y + XC → XYC (2) (catalyst w/reactants) XYC → CZ (3) (catalyst w/ products) CZ → C + Z (4) (catalyst is “alone” at end) Although the catalyst is consumed by reaction 1, it is subsequently produced by reaction 4, X + Y → Z 35 so the overall reaction is: Catalysts in Biochemistry: Enzymes- (see Ch 23) What to know for now: Globular proteins; work on highly specific substrates (and usually specific enantiomers); recyclable; lower activation energy and increase rxn rate 109 – 1020 X Usually named and classified by the rxn they catalyze Active site vs allosteric site Competitive inhibition (block active site) vs noncompetitive inhibition (bind to another site and alter the tertiary structure of enzyme, which changes the ‘shape’ of active site) Denatured by: high temp; altered pH; high salinity. Study graphs p. 618-623. Expect a test question! Feedback control: Study p. 625 Reactions of Alkenes and Alkynes Addition of H2 to alkenes and alkynes: Alkenes and alkynes react with hydrogen in the presence of a metal catalyst such as palladium to yield the corresponding saturated alkane products – called hydrogenation. **Recall unsaturated fats to saturated fats Chapter One 37 Addition of Cl2 and Br2 to alkenes: Halogenation Alkenes react with the halogens Br2 and Cl2 to give 1,2-dihaloalkane. Chapter One 38 Addition of HCl and HBr to alkenes: Alkenes react with the hydrogen bromide and hydrogen chloride to give alkyl bromide or alkyl chloride product. Chapter One 39 Markovnikov rule: In the addition of HX to an alkene, the H becomes attached to the carbon that already has the most H’s, and X becomes attached to the carbon that has fewer H’s. Chapter One 40 How does the rule work? Reaction mechanisms will show us why and how, always keeping in mind that + and - attract. *Book- p. 365 How an Alkene Addition Reaction Occurs Reaction mechanism: A description of the individual steps by which old bonds are broken and new bonds are formed. Electron flow is always from the electron-rich to the electron-poor species. The electron-rich species is a Lewis Base (must have a lone pair or double bond) and is called the “nucleophile”. The electron-poor species is a Lewis Acid (must have empty orbital) and is called the “electrophile”. *For a review of orbitals and hybridization, see links on my blog Reaction mechanisms Nucleophile= electron rich Electrophile= electron poor Carbocation: C with 3 bonds and + charge Electron flow is always from nucleophile to electrophile; from negative (anion) to positive (cation); from alkene (double bond) to cation (or partial pos charge) Draw curved arrow from n’phile to e’phile *Show www.leah4sci.com videos on following slides H O H Concepts and Terminology Carbon hybridizes sp3 when forming single bonds. But what about double bonds? Watch this video: http://leah4sci.com/sp2sp-hybridization-bondangle-molecular-geometry-tutorial-video/ . *For a review on sp3, watch this at home: http://leah4sci.com/sp3-hybridization-bondangle-molecular-geometry-tutorial-video/ *Helpful to watch all videos in this series: http://leah4sci.com/organic-chemistry-video-library/organic-chemistrybasics-to-build-a-strong-orgo-foundation/ More Concepts and Terms For alkenes (carbons w/ double bonds): Sigma bond: one bond of double bond is considered ‘single’ (s orbitals) Pi bond: is the other bond of db (p orbitals) Carbocation- carbon atom with only 3 bonds, therefore it has a positive charge Watch in class: http://leah4sci.com/alkenereactions/ Carbocation Stability Primary carbocation: carbocation is bonded to one other carbon Secondary carbocation: carbocation is bonded to to 2 other carbons Tertiary carbocation: carbocation is bonded to 3 other carbons General order of stability: 30>20>10 Hydrohalogenation: Addition of H-X *Remember- Nucleophile (e- ‘rich’ and ‘loves’ positive charge) attacks Electrophile (epoor) Steps Involved: Reaction of pi (double) bond with H+ (of H-X), concurrent separation of X- , and formation of Markovnikov carbocation intermediate. Attack on carbocation by X- to finish formation of product Reaction Mechanisms: Addition of H-X (Hydrohalogenation) Identify this mechanism – Starts with alkene, ends with single halide… X H-X Addition of H-X Step 1: Reaction of pi bond with H+ (of HX), concurrent separation of X-, and formation of carbocation intermediate. H X H + X Addition of H-X Step 2: Attack of X- to finish formation of product. X H X H Addition of H-X *Try this one by yourself, following steps of last example: Identify the mechanism… Adding a Br and an H to an alkene… H-Br Br Addition of H-X (Solution) Step 1: Reaction of pi bond with H+ (of HX), concurrent separation of X-, and formation of carbocation intermediate. H Br H + Br Addition of H-X - Again Step 2: Attack of X- to finish formation of product. Br H Br H Addition of HX – One more to try: Identify the mechanism (show steps): *Adding a chloride (and a H, which is not drawn in) to an alkene. Remember Markovnikov’s rule! H-Cl Cl Addition of HX – Solution Step 1: React the pi bond with H+ (of HX), separate off the X-, form the more substituted carbocation intermediate. H H Cl + Cl Addition of HX – Solution Step 2: Attack of X- to finish formation of product. H Cl Cl H Classwork- Do now. I will be around to check Do problem 12.6 a and b from p. 364 (showing steps of mechanism) and 12.7 (p. 366) Homework: Complete worksheet Homework Problems (from worksheet) 1. Show the 2 step mechanism for the reaction of 3-hexene with hydrochloric acid and name product. 2. Show the 2 step mechanism of 2methyl-1-pentene with hydrobromic acid. Name product 3. Show the 2 step mechanism of ethylcyclohexene with hydroiodic acid. Name product. Addition of water to alkenes: Hydration Alcohol is produced on treatment of the alkene with water in the presence of a strong acid catalyst, such as H2SO4. Markovnikov’s rule can be used to predict the product when water adds to an unsymmetrically substituted alkene. Hydrated alkenes produce alcohols. Chapter One 59 Try this one (overall reaction only): Draw a structural formula for the alcohol formed by the hydration of 2-methylpropene. *Do NOT have to name specific alcohols on this test One of you will come to board to show the result Answer: 2-methyl-2-propanol (do not have to name it specifically for this test, but know it’s an alcohol with hydroxyl functional group) Example #2 of Hydration: What is the mechanism? *Go back and review the section on catalysts CH3 CH=CH2 Propene + H2 O H2 SO4 OH H CH3 CH-CH2 2-Propan ol NOTE- you will not have to name specific alcohols on this test! Only identify them as alcohols ** Acid-Catalyzed Hydration Steps Involved: Reaction of pi bond with H+ (acid catalyst!) resulting in Markovnikov carbocation formation (note- the H+ is from a strong acid, like HCl, H2SO4, etc) Addition of H2O (this is where the OH is coming from) Removal of extra proton (H+) to finish formation of –OH. Mechanism for Hydration (*Show leah4sci video on FC; show leah4sci.com video of partial resonance?- Video 7: https://www.youtube.com/watch?v=zm1-gUxKr3g ) Video 6: https://www.youtube.com/watch?v=zm1-gUxKr3g Step 1: H2SO4 CH3 CH=CH2 + H+ *H+ is from H2SO4 H CH3 CHCH2 A 2° carb ocation intermediate + Step 2: CH3 CHCH3 H O+ CH3 CHCH3 + :O-H H H O+ CH3 CHCH3 An oxonium ion : Step 3: H : *What is FC on O? : + : H :OH *What is FC on O? CH3 CHCH3 + H+ *Bonds w/ HSO4- to 2-Propan ol regenerate catalyst Try this one: Show the mechanism of the hydration of 2-methyl-1-butene. I will call someone up to the board Be sure to Review Exp 12.9 in book and do problem 12.9 Alkene Polymers Polymers: A large molecule formed by the repetitive bonding together of many smaller molecules called monomers. Many simple alkenes undergo polymerization reaction when treated with the proper catalyst. 66 Polymerization Polymerization is a VERY important reaction of alkenes polymer: Greek: poly, many and meros, part monomer: Greek: mono, single and meros, part nCH2 =CH2 Ethylene initiator (polymerization) CH2 CH2 n Polyethylene Polymerization Use parentheses around the repeating monomer unit Subscript, n, indicates that this unit repeats n times Show that a polymer chain can be reproduced by repeating the enclosed structure in both directions Example: section of polypropene (polypropylene) monomer un its show n in red n CH3 CH3 CH3 CH3 CH2 CH-CH2 CH-CH2 CH-CH2 CH CH3 CH2 CH n Part of an extended polymer chain The repeating un it Monomer Formula CH2 =CH2 Common N ame ethylene CH2 =CHCH3 propylene CH2 =CHCl CH2 =CCl2 Polymer N ame(s ) and Common Uses polyethylen e, Polyth ene; break-resistan t containers polypropylene, Hercu lon; textile and carp et fib ers vinyl chlorid e poly(vinyl ch loride), PV C; cons truction tubing 1,1-dichloropoly(1,1-d ichloroethylene); ethylene Saran Wrap is a cop olymer w ith vinyl chlorid e CH2 =CHCN acrylon itrile CF2 =CF2 tetrafluoroethylene polyacrylonitrile, Orlon; acrylics and acrylates polytetrafluoroethylene, PTFE; Teflon , nonstick coatin gs CH2 =CHC6 H5 CH2 =CHCOOC2 H5 styrene ethyl acrylate polystyrene, S tyrofoam; insulation poly(eth yl acrylate); latex paints CH2 =CCOOCH3 CH3 methyl methacrylate poly(methyl methacrylate), Lucite, Plexiglas; glass s ubs titu tes Note: I will only require you to know a couple of these for this test……most notably, ethylene and propylene. Maybe 1 or 2 more…..vinyl chloride? Anyone doing doing a polymerization for Herbfest? If so, be sure and include the monomer in your lab report/paper and on your board. If your active ingredient is a terpene, find out which enantiomer (R or S) or if it’s a racemic mixture (combination of the 2). You will understand what these both mean SOON! Polymerization of vinyl chloride (chloroethene) to PVC Polyethylene Low-density polyethylene (LDPE) Highly branched polymer, so chains do not pack well—weak London Force interactions Softens and melts above 115°C Primarily used for packaging for trash bags High-density polyethylene (HDPE) Little branching, so chains pack well--London dispersion forces between them are stronger Higher melting point and stronger than LDPE Used for squeezable jugs and bottles FYI-Codes for Plastics (don’t expect you to know) 5 PP Polymer poly(eth ylen e terephthalate) high-den sity polyethylen e poly(vinyl ch loride), PV C low -density polyethylen e polypropylene 6 PS polystyrene 7 all other p lastics Cod e 1 PET 2 HD PE 3 V 4 LD PE Common Uses soft drin k bottles, hous ehold ch emical b ottles , films, textile fib ers milk and w ater jugs, grocery bags, sq ueezable bottles sh amp oo bottles, pip es, show er curtains, vinyl siding, w ire in sulation, floor tiles sh rin k w rap , tras h and grocery bags, san dw ich b ags , squ eeze bottles plas tic lids, clothin g fibers, bottle cap s, toys , diaper linings styrofoam cu ps, egg carton s, disp os able uten sils, packaging materials, app lian ces various Nomenclature Review: Name These CH3 C H CH3 C Cl CH2 CH3 CH2 CH3 C C H Cl Ch 13: Aromatic Compounds and Benzene Aromatic compounds contain benzene. Benzene, C6H6 , is represented as a six carbon ring with 3 alternating double bonds. Two possible can be drawn to show benzene in this form. H H H H H H H H H H H H Aromatic Compounds and the Structure of Benzene In the early days the word aromatics was used to described many fragrant molecules isolated from natural sources. Today the term aromatic is used to describe benzene like molecules. Benzene is a flat, symmetrical molecule with the molecular formula C6H6. It has alternating three carbon-carbon double and three single bonds. Benzene’s relatively lack of chemical reactivity is due to its structure. Unlike alkenes, benzene does not undergo addition reactions There are two possible structures with alternating double and single bonds. Experimental evidence suggest that all six carbon-carbon bonds in benzene are identical. The properties, including the above one, of benzene can only be explained by assuming that the actual structure of benzene is an average of the above two possible equivalent structures-known as resonance. Simple aromatic compounds like benzene are non-polar, insoluble in water, volatile, and flammable. Unlike alkenes, several aromatic hydrocarbons are toxic. Benzene itself is implicated as a cancer causing chemical. Aromatic Compounds in Nature and Health Many aromatic compounds are common in nature and in medicine. CHO COOH COOCH3 CH3 CH3 CH3CHCH2 CHCOOH OCH3 OH Aspirin Vanillin Ibuprofen Naming Aromatic Compounds Aromatic compounds are named with benzene as the parent chain. One side group is named in front of the name benzene. - No number is needed for mono-substituted benzene since all the ring positions are identical. CH3 methylbenzene (toluene) Cl chlorobenzene Naming Aromatic Compounds When two groups are attached to benzene, the ring is numbered to give the lower numbers to the side groups. The prefixes ortho (1,2), meta (1,3-) and para (1,4-) are also used. O,M,P Cl CH3 Cl CH3 Cl CH3 1,2-dimethylbenzene 1,3-dichlorobenzene (ortho-dimethylbenzene) (meta-dichlorobenzene) (para-chloromethylbenzene) M-dichlorobenzene P-chloromethylbenzene O-dimethylbenzene 1-chloro-4-methylbenzene Some Common Names Some substituted benzene rings also use a common name. Then naming with additional more side groups uses the ortho-, meta-, para- system. CH3 OH CH3 Cl Toluene (Methylbenzene) meta-chlorotoluene (meta-chloromethylbenzene) phenol (hydroxybenzene) Many substituted aromatic compounds have common names in addition to their systematic names. Learning Check Select the names for each structure: Cl a. Chlorocyclohexane b. Chlorobenzene c. 1-chlorobenzene CH 3 CH 3 a. Meta-xylene b. Meta-dimethylbenzene c. 1,3-dimethylbenzene Learning Check Write the structural formulas for each of the following: A. M-dichlorobenzene B. Ortho-chlorotoluene Attached Groups (*only know the ones on the left) Phenyl 4-phenyl-1-butene Benzyl Benzyl alcohol Nitro -NO2 Refer to nomenclature packet for order of priority! 2,4,6-trinitrotoluene Reactions of Aromatic Compounds Unlike alkenes which undergo addition reactions, aromatic compounds undergo substitution reactions. That is, a group y substitutes for one of the hydrogen atoms on the aromatic ring. Some examples are given below: 88 Nitration reaction: Substitution of a nitro group (NO2-) for a ring hydrogen. Halogenation reaction: Substitution of a halogen (Cl or Br) for a ring hydrogen. Sulfonation reaction: Substitution of a sulfonic (SO3H-) group for hydrogen. (*not on this test) a ring 89 Learning check: Show the chlorination of benzene * Remember……benzene undergoes substitution, like alkanes Chapter Summary Alkenes contain carbon-carbon double bonds. Alkynes contain carbon-carbon triple bonds. Aromatic compounds contain six carbons in a ring arrangement with three double and three single bonds alternating between carbon atoms. Alkenes are named using the family ending – ene, while the alkynes use the family ending – yne. Alkenes and alkynes generally undergo addition reactions and aromatic compounds generally undergo substitution reactions. Reaction mechanism: A description of the individual steps by which old bonds are broken and new bonds are formed. 91