* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Detection of Single Microtubules in Living Cells
Survey
Document related concepts
Chromatophore wikipedia , lookup
Cell encapsulation wikipedia , lookup
Spindle checkpoint wikipedia , lookup
Endomembrane system wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell culture wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell growth wikipedia , lookup
Cytoplasmic streaming wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
List of types of proteins wikipedia , lookup
Transcript
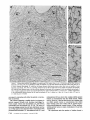
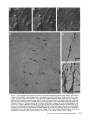
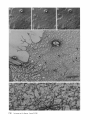
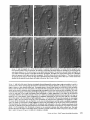
Detection of Single Microtubules in Living Cells: Particle Transport Can Occur in Both Directions along the Same Microtubule I. H. HAYDEN and R. D. ALLEN Department of Biological Sciences, Dartmouth College, Hanover, New Hampshire 03755 ABSTRACT Video-enhanced contrast/differential interference-contrast microscopy was used in conjunction with whole mount electron microscopy to study particle transport along linear elements in fibroblasts. Keratocytes from the corneal stroma of Rana pipiens were grown on gold indicator grids and examined with video microscopy. Video records were taken of the linear elements and associated particle transport until lysis and/or fixation of the cells was completed. The preparations were then processed for whole mount electron microscopy. By combining these two methods, we demonstrated that linear elements detected in the living cell could be identified as single microtubules, and that filaments as small as 10 nm could be detected in lysed and fixed cells. The visibility of different cytoplasmic structures changed after lysis with many more cellular components becoming visible. Microtubules became more difficult to detect after lysis while bundles of microfilaments became more prominent. All particle translocations were observed to take place along linear elements composed of one or more microtubules. Furthermore, particles were observed to translocate in one or both directions on the same microtubule. Cytoplasmic transport is a process of ubiquitous occurrence in eucaryotic cells and of fundamental importance to cell function. One manifestation of cytoplasmic transport, the translocation of optically detectable organdies and vesicles (collectively called particles), has been extensively studied (20, 27). Microtubules have long been believed to play an important role in particle transport in such diverse cells as chromatophores (4, 22), neurons (15), tissue culture cells (13), and in numerous protists, e.g., the foraminefer, Allogromia (34, 35). These cells possess impressive arrays of cytoplasmic microtubules, the presence and orientation of which have been shown by electron (4, 5, 13, 34, 36) and immunofluorescence (9) microscopy to be consistent with the hypothesis that particle motion occurs along them. Furthermore, microtubule depolymerizing drugs have been shown to inhibit particle motion in all these cells (13, 17, 25, 33). Until recently, the major limitation of particle transport studies had been the inability to detect and identify cytoplasmic microtubules in the living cell. Video-enhanced microscopy has now made it possible to observe microtubules in vitro in a slurry (1) or in a living cell (18). In a previous study, using Allen video-enhanced contrast/differential interTHE JOURNAL OF CELL BIOLOGY • VOLUME 99 NOVEMBER 1984 1785-1793 © The Rockefeller University Press • 0021-9525/84/11/1785/09 $1.00 ference-contrast (AVEC-DIC) t microscopy in conjunction with immunofluorescence microscopy, we demonstrated directly that particles move in keratocytes along linear elements composed of microtubules (18). The particles displayed a number of interesting behaviors including motion in either direction along the same microtubular linear element. However, this study was limited in that the number of microtubules or other cytoskeletal elements making up a linear element could not be determined. Thus, it was possible that a particle could move in one direction along one microtubule in a linear element composed of microtubules and on another microtubule in the reverse direction. In the present study, we observed particles moving along linear elements in keratocytes preceding fixation and preparation for whole mount electron microscopy. We show that by comparing the video record to the corresponding whole mount electron micrographs, one can determine the numbers and kinds of cytoskeletal elements that make up a light ~Abbreviation used in this paper: AVEC-DIC microscopy, Allen video-enhanced contrast/differential interference-contrast microscopy. 1 785 microscopic linear element. We demonstrate that A V E C - D I C can detect (a) single microtubules in living cells, a n d (b) filaments as small as 10 n m in cells that have been lysed a n d fixed. Furthermore, particle translocations can occur in one or both directions along the same microtubule. MATERIALS AND METHODS Corneasfrom Rana pipiens were removedand the epitheliumand endothelium were stripped away. The remaining stroma that contained the keratoeyteswas placed between two number 0 coverslipsin petri dishes and grown at 24"C in a 5% CO2 atmosphere, conditions under which the keratoeytesmigrated from the stroma onto the coverslips.The cells were maintained in Eagle's minimal essential medium supplemented to a final concentration of 10% fetal calf serum, 0.I mM nonessential amino acids, 2.0 mM L-glutamine, and 1% antibiotic-antimycoticsolution (100 U/ml penicillin, 100 #g/ml streptomycin, 0.5 ~,g/mlFungizone). Alt media components were supplied by Gibco Laboratories, Grand Island, NY. Gold London Finder grids (Ladd Research Industries, Inc., Burlington,VT) were Formvar- and carbon-coated,and sterilizedby exposure to ultravioletlight. The keratocyteswere collectedfrom the coverslips with 0.25% trypsin made up in Ca+* and Mg+* free Earle's balanced salt solution (Gibco Laboratories) and seeded onto the prepared electron microscope grids. In preparation for light microscopy,a coverslipwas fastenedto a slideholder with vaseline-lanolin-paraffin(1:1:1), and the grids with attached cells were placed in a drop of medium on the coverslip.A chamber was formed by laying a clean coverslipon top of strips of Scotch tape that ran the length of two sides of the bottom coverslip. These two sides were sealed with vaseline-lanolinparaffin (1:1:1). The preparations were examined with AVEC-DIC (1, 2) microscopy using an inverted Zeiss Axiomat (Carl Zeiss, Inc., Oberkochen, Wuerttenberg,West Germany) equipped with a 100 x/1.3 NA planapochromatic objective.The bias re'tardationwas set to h/9. The image was projected either onto a C-10004)1 binary computer-compatiblechalnicon video camera driven by a model 1440 Poly!aroeessorFrame Memory (Hamamatsu Systems Inc., Waltham, MA), or onto a Chalmicon video camera driven by a newer device, the C 1966 AVEC/VIM Photonic Microscope System Image Processor (Photonic Microscopy,Inc., Chicago, IL). Once the video records of the cells were taken, the preparations were processed for whole mount electron microscopy.The cells were either lysed and then fixed, lysed and fixed simultaneously,or fixed without lysis. Lysis and/or fixationmedium was added to one open sideof the coverslippreparation and drawn through by placing filter paper strips at the other open side. The microtubulestabilizingbuffer used for lysisand/or fixationconsistedof 25 mM PIPES (pH 6.9), 1.0 mM EGTA, 1% polyethyleneglycol 6000, and 0.5 mM MgCI. This buffer contained 0.5% Triton X-100 and 1.0% glutaraldehydefor lysis and fixation, respectively.Video records were taken of lysis and fixation. After fixation for 30 min in glutaraldehyde, the preparations were removed from the light microscope, washed with microtubule stabilizingbuffer, and postfixed for 1 rain with 1.0% osmium in 100 mM Cacodylatebuffer (pH 6.0, 4"C). The preparations were then washed with distilled water, dehydrated through a graded ethyl alcohol series, and critical point dried. At the 70% alcohol dehydration stage, the preparations were stained for 1 rain with 0.5% uranyl acetate in 70% alcohol. The cellswere examinedwith a JEOL 100 CX electronmicroscopeoperating at 100 kV, and the whole mount electron mierographs correspondingto the video records were obtained. RESULTS All structures visible in the light micrographs of living cells were referred to in general terms as either linear elements or particles. After lysis a n d / o r fixation, the linear elements were subsequently identified from the corresponding whole m o u n t electron micrographs o n the basis of size a n d organization. According to convention, the 6 - n m filaments were referred to as microfilaments (MFs), a n d the 10-nm filaments as intermediate filaments (IFs). The microtubules (MTs) were identified both o n the basis o f their size 0 8 - 2 0 n m ) a n d their characteristic pattern in the cell. (See figure legends for a more detailed description of the results.) In the flattened peripheral regions of these cells, various linear elements could be seen (Fig. 1 a). In preparation for whole m o u n t electron microscopy, the cells were lysed a n d fixed while they r e m a i n e d in focus in the light microscope. 1786 THE IOURNAL OF CELL BIOLOGY- VOLUME 99, 1984 The living cell in Fig. I a is shown again in Fig. I b after lysis a n d fixation in a microtubule stabilizing solution that contained 0.5% T r i t o n X-100 a n d 1.0% glutaraldehyde. The replacement of the soluble c o m p o n e n t s of the cell with the lysis m e d i u m led to a n increase in contrast for all linear elements visible in the living cell, a n d caused m a n y other cellular c o m p o n e n t s to b e c o m e visible for the first time. In fact, one linear element became evident only after lysis. O n e result of this increase in contrast was that some of the linear elements pointed out in the living cell (Fig. 1 a) were more difficult to detect. This is because the increased n u m b e r of visible cellular c o m p o n e n t s after lysis tends to obscure, partially or totally, some linear elements. Whole m o u n t electron miroscopy was used to identify the linear elements seen in the living a n d lysed cells. Fig. 2 a is a low magnification electron micrograph of the same region shown in the light microscope in Fig. 1. The linear element that b e c a m e apparent only u p o n lysis was a n intermediate filament (Fig. 2, a a n d c), a n d two linear elements that became more evident u p o n lysis corresponded to bundles of micro fila m e n t s (Fig. 2 a). O n e linear element, visible in the living cell b u t totally obscured in the lysed cell, was composed of two microtubules (Fig. 2 a). However, the two curvilinear elements pointed out in the live a n d lysed cell (Fig. 1, a a n d b) are single microtubules (Fig. 2, a a n d b). Thus, single microtubules are detected in the living cell by AVEC-DIC microscopy, while a 10-nm filament could be detected only in the lysed cell. W e observed particles translocating along linear elements composed of microtubules in living cells. In Fig. 3, a a n d b, a particle is shown m o v i n g d o w n a p r o m i n e n t linear element. This particular cell was then lysed for 30 s with 0.5% T r i t o n X-100 in a microtubule stabilizing solution before fixation with 1.0% glutaraldehyde. Once again, u p o n lysis m a n y more cellular c o m p o n e n t s became apparent, thus obscuring the linear element the particle m o v e d along (Fig. 3 c). Also, some of the less evident linear elements in the living cell became the most visually p r o m i n e n t elements in the lysed cell. The corresponding whole m o u n t electron micrograph d e m o n strates these latter elements to be bundles of microfilaments (Fig. 3, d a n d f ) , while the linear element along which particles translocated in the living cell is composed of two microtubules (Fig. 3, d a n d e). Particles were also observed translocating along linear elements composed of single microtubules. In Fig. 4, a a n d b, a particle is shown m o v i n g along one of three parallel linear elements. A total of four particles translocated in the same direction along the lower two linear elements. These linear elements were identified as single microtubules in the corresponding whole m o u n t electron micrographs (Fig. 4, d a n d e). I n this preparation, the cell was fixed without lysis in a microtubule stabilizing solution that contained 1.0% glutaraldehyde. In this case, the microtubules only became obscured by other cellular c o m p o n e n t s in a region of vesiculation; otherwise, the microtubules appeared to retain their relationships to the rest o f the cell to the extent that they r e m a i n e d the most p r o m i n e n t linear elements in the region. Finally, in another preparation, a particle was observed to translocate in both directions along a linear element (Fig. 5 a e). This preparation was lysed a n d fixed in a microtubule stabilizing buffer that contained 0.5% T r i t o n X-100 a n d 1.0% glutaraldehyde. The corresponding whole m o u n t electron micrograph shows that this linear element consisted of a single microtubule (Fig. 6). This was one of two preparations in FIGURE 1 AVEC-DIC images demonstrating linear elements in a living cell grown on a gold London Finder grid. (a) The living cell with arrowheads and arrows pointing out various linear elements. (b) The same region after lysis and fixation. After lysis there was an increase in contrast that caused many cellular components to become evident for the first time. In fact, a linear element became visible (small arrows) that was not seen in the living cell. Also, the two linear elements pointed out in the living cell by the large arrows gained in contrast to the extent that they became more evident after lysis. On the other hand, the increased number of visible cellular components made it more difficult to detect some of the linear elements. For example, the curvilinear elements (black arrowheads) and one linear element (white arrowheads) seen in the living cell became partially and totally obscured, respectively, by the many cellular components made visible after lysis. Bar, 2.0 v,m. x 5,000. which transport in both directions along a single microtubule was documented. Thus, particles can not only translocate along single microtubules, but they can also do so in either direction. DISCUSSION Particle Transport We have used video microscopy in conjunction with whole mount electron microscopy to demonstrate that linear elements detected in the living cell can subsequently be identified as single microtubules. Furthermore we detected and identified filaments as small as 10 nm in diameter in lysed cells. The main significance of these observations is that some of the functions of cytoplasmic microtubules can now be studied directly. Using this approach to study the role ofmicrotubules in particle transport, we have demonstrated that particles can move in both directions on the same microtubule. In a previous study with keratocytes (18), we showed using AVEC-DIC microscopy in conjunction with immunofluorescence microscopy, that particles are transported exclusively along linear elements composed of microtubules. Particles were also observed to undergo a number of interesting behaviors including motion in either direction on the same microtubular linear element. However, it was not possible to determine the number of microtubules making up a linear element. Particle motion in both directions along the same micro- tubular linear element is of interest when one considers the possibility that (a) the structural polarity of microtubules is translated into a functional polarity, and (b) dynein, an ATPase mechanoenzyme, (14) is the mechanochemical transducer responsible for particle translocation. Does the structural polarity of microtubules manifest itself as a functional polarity? Some models for mitosis assume that the direction of polarity of the microtubules plays an important part in chromosome motion (23, 24). Our work showing particle motion in both directions along the same microtubule indicates, at least for particle transport in keratocytes, that the structural polarity of the microtubules does not dictate the direction of motion. Also, recent studies with nerve axons (7, 11, 19), melanophores of the angel fish (10), and axopodia of Echinosphaerium (10) have shown most of the microtubules to be of the same polarity though bidirectional particle motion occurs in these cells. Whether dynein could be the mechanochemical transducer responsible for particle translocation also hinges on questions of polarity. Dynein is an asymmetric molecule that has been used to determine the structural polarity of microtubules (16). Ciliary dynein is attached to the A-subfiber by its A-end (32), and it interacts with the B-subfiber via its B-end which contains the ATPase activity. In a simple microtubule-dyneinparticle hypothesis, the dynein could presumably be attached to either the microtubule or particle by its A-end. Under the proper conditions the dynein would then be able to generate HAYOEN AND ALLEN Particle Transport along Single Microtobules I 787 FIGURE 2 Whole mount electron micrographs, corresponding to the region shown in Fig. 1, demonstrating that linear elements detected in the living cell can be identified as single microtubules. In a the linear element that became evident only after lysis is a 10-nm, filament (arrowheads, IF), while the two linear elements that became more evident after lysis are bundles of 6-nm filaments (arrowheads, MFs). The two curvilinear elements seen in the live and lysed cell are single microtubules (arrowheads, MT), and the linear element seen in the live cell but obscured in the lysed cell is composed of two microtubules (arrowheads, 2 MTs). A higher magnification of the microtubule defined by the small arrowheads (MT) in a is shown in b. A higher magnification of the intermediate filament defined by the small arrowheads (IF) in a is shown in c. Bars: (a) 2.0 /~m; (b and c) 0.2 pro. (a) x 11,000; (b and c) x 55,000. movement by interacting with either the particle or microtubule via its B-end. The evidence suggesting a possible dynein involvement in particle transport includes work showing cross-bridges between synaptic vesicles and microtubules (21), and between mitochondria and microtubules (26, 29, 30). The nature of this cross-bridging material has not been determined, nor has any ATPase activity been associated with it. Experiments with permeabilized fibroblasts (12), neuroblastoma cells (31), and 1788 THE JOURNAL OF CELL BIOLOGY • VOLUME 99, 1984 melanophores (8) have shown that vanadate inhibits particle motion at concentrations that also inhibit dynein ATPase activity. However, it also has been shown that vanadate does not inhibit particle motion at concentrations that inhibit ciliary beating in the same oviduct cell (6). Erythro-9-[3-(2hydroxymomyl)]adenine, another dynein ATPase inhibitor, inhibits particle motion in intact erythrophores (3) and melanophores (8). Our experiments raise the question of whether dynein is FIGURE 3 Light micrographs and whole mount electron micrographs demonstrating particle motion along a linear element composed of microtubules. A particle (large arrows) is moving down a prominent linear element (small arrows} in the live cell in a and b. The lysed and fixed cell is shown in c. Here again, many cellular components became visible upon lysis; this tends to obscure the linear element the particles moved upon. A portion of this linear element is still visible (small arrow), while some linear elements (arrowheads in b and c) became much more prominent after lysis. The corresponding electron micrograph of this cell region is shown in d. The linear element that the particle moved along is composed of two microtubules (large and small arrows, MTs). A higher magnification micrograph of the region of these two microtubules defined by the small arrows in d is shown in e. The microtubules in this region are still detectable in the light microscope after lysis (c) because the area immediately surrounding them contains very little material. The linear elements that became more prominent after lysis are composed of microfilaments (d and f, large and small arrowheads, MFs). A region of one of these bundles of filaments defined by the small arrowheads in d is shown in/'. Bars: (c and d) 2.0/zm; (e and f) 0.2 #m. (c) × 4,300; (d) x 9,300; (e and f) x 60,000. HAYDENAND ALLEN Particle Transport along Single Microtobules 1789 1 790 THE JOURNAL OF CELL BIOLOGY - VOLUME 99, 1984 FIGURE 5 Light micrographs of a cell in which motion of a particle (white arrowheads, P) in either direction along the same linear element (white arrows) was observed. The particle is moving down the linear element (a), pausing (b), and returning up the same linear element (c-e). The cell is shown in e just before lysis and fixation. The particle is seen just before reaching the cell mass (black arrowhead) present in both light and electron micrographs. The black arrow at the bottom points out a filopodium that is also present in both light and electron micrographs. The cell is shown after lysis and fixation in f. The linear element that the particle moved along (as well as the other linear elements) became much harder to detect after lysis. A section of the linear element that the particle moved along is still visible at the arrow. Bar, 2.0 #m. x 12,000. FIGURE 4 Light and whole mount electron micrographs demonstrating particle motion along a single microtubule. (a and b) A particle (arrows) is shown moving along the lowermost of the three linear elements labeled by the black arrowheads. The same region is shown in c after fixation without lysis. The particle shown in (a and b) had moved out of the field of view just before fixation. Note that there was some vesiculation at the lower left (white arrowheads). The letter B (c and d) indicates holes in the plastic that are useful as landmarks when comparing the light micrographs to the whole mount electron micrographs. The whole mount electron micrograph corresponding to the light micrographs (a-c) is shown in d. Note that the three linear elements seen in the live and fixed cell are single microtubules (black arrowheads). A higher magnification of the region of the lowermost microtubule defined by the small arrowheads is shown in e. Other non-microtubular elements closely surround it and may run parallel to it for a short distance. Some arrangements of cytoskeletal components may have the same width as a microtubule, and so they appear to be microtubules at high magnification. In e, -1 cm to the left of the second arrowhead, the microtubule appears to split in two and continue to the left as two microtubules. By using the lower magnification micrograph (d) to follow the course of the microtubule, one sees that there is only one microtubule present and that another cytoskeletal component, only a few tenths of a micrometer in length, happens to intersect the microtubule just to the left of the second arrowhead (e). This demonstrates the importance of using both the microtubule width and pattern in the cell to identify microtubules. In the living cell, two particles moved along the bottommost microtubule and two particles moved along the microtubule immediately above it. In this preparation, the microtubules remained as very prominent elements after fixation without lysis. They only became obscured in the region of vesiculation where an increase in contrast caused other cellular components to become visible. Bars: (c and d) 2.0/zm; (e) 0.2 #m. (c) x 3,800; (d) x 20,000; (e) x 90,000. HAYDENAND ALLEN Particle Transportalong Single Microtobules 1791 FIcueE 6 (a) Whole mount electron micrograph, corresponding to the cell region in Fig. 5, demonstrates that the linear element that the particle moved along in either direction is a single microtubule (white arrows). The letters ae correspond to the sequence of particle positions seen in the living cell with the white arrowheads showing the direction of motion. The particle reversed directions at b and was observed at e just before reaching the cell mass shown here by the star (corresponding to the area shown by the black arrowhead in Fig. 5e). The filopodium noted in Fig. 5e is shown at the lower right (black arrow). The region of the cell in which the particle was last observed (Fig. 5e) is shown at higher magnification in b. The star labels the cell mass seen in Fig. 5 and Fig. 6a. The arrows point out the microtubule that the particle moved along. Note that other cytoskeletal components closely surround the microtubule. Bars: (a) 1.0/~m; (b) 0.1 tLm. (a) x 22,000; (b) x loo,0oo. still a candidate for the mechanochemical transducer. If the structural polarity of the dynein-microtubule interaction also dictates a functional polarity, then our studies would appear to rule out a dynein involvement in particle transport. However, there is some confusion on the point of dynein-microtubule polarity. In one study with microtubules assembled in 1792 THE JOURNAL OF CELL BIOLOGY • VOLUME 99, 1984 vitro (16), dynein was shown to cross-bridge microtubules only when they were of the same polarity; in another study dynein was shown to cross-bridge ciliary microtubule doublets of the same and opposite polarity (37). The doublets were still capable of sliding relative to one another upon addition of ATP. Resolution of this dynein-microtubule polarity question is necessary to evaluate the significance of particle transport in both directions along the same microtubule. It is possible that a microtubule-dynein interaction may not be involved in particle transport. It may be that microtubules only serve to define the paths particles may follow through the cytoplasmic matrix and that some other mechanochemical transducer is responsible for particle transport. We can only say from our light microscopy studies that particles always travel in optical contact with microtubules (i.e., within 200 nm). However, the corresponding electron micrographs show that the microtubules are surrounded by as yet unidentiffed elements which are much closer than 200 nm to the microtubules (Figs. 4 e and 6). It is possible that these elements are also involved in particle transport. Clues to the identity of any mechanoenzyme that may be involved in particle transport remain elusive. We plan to approach this problem by preserving the particles so that the particle-microtubule interactions can be determined. We hope that by correlating known particle behaviors at the time of fixation to the corresponding whole mount electron micrographs, we will gain further insight into the problem of particle transport. Cytoplasmic Matrix Some interesting observations of the present study came from comparing the living and lysed cells to one another and to the whole mount electron micrographs. Although the microtubules were the most prominent linear dements in the living cell, linear elements composed of microfilaments were more prominent in the lysed cell. All the linear elements gained in contrast because the lysis removed the Triton Xsoluble components of the cell, thereby increasing the refractive index gradient between linear elements and background. This increase in contrast caused many cellular elements to become evident for the first time, thus obscuring the microtubular linear elements. However, this does not explain why bundles of microfilaments, which were less obvious in the living cell, became much more evident than the microtubules in the lysed cell. After looking at the whole mount electron micrographs, we predicted that the bundles of microfilaments would be more prominent than the microtubules in the DIC image, as indeed they are in the lysed cell. One possible explanation for the low contrast of the microfilament bundles in the living cells is that they are surrounded by a higher concentration of Triton X-soluble material, such as G-actin, than the microtubules. It has been shown that Triton X removes 60-70% of the total cellular protein from tissue culture cells, with actin as the major soluble component (28). Removal of G-actin and any Triton X-soluble F-actin thus would result in the great increase in contrast of the microfilament bundles we observe in the lysed cell. In the future, we plan to expand this work by comparing the optical properties, such as phase gradients (DIC microscopy) and birefringence (polarized light microscopy), of various components of the cytoplasmic matrix in both the riving and/or lysed and fixed cell to the corresponding whole mount micrographs. This type of study may help interpret the behaviors that the components of the cytoplasmic matrix exhibit when their functions are studied with video microscopy. The authors are grateful to Roger Sloboda and George Ruben for their helpful discussion and criticism of the work. We particularly thank Kenneth Orndorff for his photographic work. This research was supported by a grant from the National Institute of Neurological and Communicative Disorders and Stroke (NS 19962) to R. D. Allen. Received for publication 8 May 1984, and in revised form 26 July 1984. REFERENCES 1. Allen, R. D., and N. S. Allen. 1983. Video-enhanced microscopy with a computer frame memory. J. Microsc. (Oxf.). 129:3-17. 2. Allen, R. D., N. S. Allen, and J. L. Travis. 1981. Video-enhanced constrast, differential constrast (AVEC-DIC) microscopy: a new method capable of analyzing microtubulerelated motility in the teticulopodial network of Allogromia laticollaris. Cell Motility. 1:291-302. 3. Bcckerle, M. C., and IC R. Porter. 1982. lnhibitors ofdyncin activity block intracelhilar transport in erythropbores. Nature (Lond.). 295:701-703. 4. Bikle, D., L G. Tilney, and K. R. Porter. 1966. Microtubules and pigment migration in the melanophores of Fundulus heteroclitus L. Protoplusma. 61:322-345. 5. Breuer, A. C., C. N. Christian, M. Henkart, and P. G. Nelson. 1975. Computer analysis of organelle translocations in primary neuronal cultures and continuous cell fines. J. Cell Biol. 65:562-576. 6. Buckley, I. K. 1983. Ciliary but not saltatory movements are inhibited by vanadate microinjected into living cultured cells. Cell Motility. 3:167-185. 7. Burton, P. R., and J. L Paige. 1981. Polarity ofaxoplasmic microtubules in the olfactory nerve of the frog. Proc. Natl. Acad. ScL USA. 78:3269-3273. 8. Clark, T. C., and J. L. Rosenhaum. 1982. Pigment particle translocation in dctergentpermeab'flized melanophores of Fundulus heterc~litus. Proc. Natl. Acad. Sci. USA. 79:4655--4659. 9. Couchman, J. R., and D. A. Rees. 1982. Organelle-cytoskeleton relationships in fibroblasts: mitochondria, Golgi apparatus and endoplasmic reticulum in phases of movement and growth. Eur. J. Cell Biol. 27:47-54. 10. Euteneuer, U.,and J. R. McIntosh, 1981. Polarity of some motility related microtubules. Proc. Natl. Acad. Sci. USA. 78:372-376. I 1. Filliatreau, G., and L. Di Giamberardina. 1981. Microtubules polarity in myelinated axons as studied after decoration with tubulin. Biol. Cell. 42:69-72. 12. Forman, D. S. 1982. Vanadate inhibits saltatory organelle movement in a pcrmeab'dized cell model. Exp. Cell Res. 141:139-147. 13. Freed, L J., and M. M. Lebowitz. 1970. The association of a class ofsultatory movements with microtubules in cultured cells. J. Cell Biol. 45:334-354. 14. Gibbous, I. R., and A. J. Rowe. 1965. Dynein: a protein with ATPase activity from (~lia. Science(Wash. DC). 149:424--426. 15. Grafstein, B., and D. S. Forman. 1980. Intracellular transport in neurons. Physiol. Rev. 60:1167-1283. 16. Haimo, L. T., B. R. Telzer, and J. L. Rosenbaum. 1979. Dynein binds to and crossbridges cy~plasmic microtubules. Proc. Natl. Acad. Sci. USA. 76:5759-5763. 17. Hammond, G. R., and R. S. Smith. 1977. Inhibition of the rapid movement of optically detectable axonal particles by colchicine and vinblastine. Brain Bes. 128:227-242. 18. Hayden, J. H., R. D. Goldman, and R. D. Allen. 1983. Cytoplasmic transport in keratocyte: direct visualization of particle translocation along microtubules. Cell Motility. 3:1-19. 19. Heidemann, S. R., J, M. Landers, and M. A. Hamborg. 1981. Polarity of axonal microtubules. J. Cell Biol. 91:661-665. 20. Hyams, J. S., and H. Stebbings. 1979. Microtubule associated cytoplasmic transport. In Mierotubules. K. Roberts and J. S. Hyams, editors. Academic Press, Inc., London. 487530. 21. Jartfors, U., and D. S. Smith. 1969. Association between synaptic vesicles and neurotubules. Nature (Lond. ). 224:710-711. 22. Luby-Phelps, K. J., and M. Schliwa. 1982. Pigment transport in chromatophores: a model system for intraceUular transport. In Axoplasmic Transport. D. G. Weiss, editor. Springer-Verlag, Berfin Heidelberg. 15-26. 23. Margolis, R., L. Wilson, and B. Kiefer. 1978. Mitotic mechanism based on intrinsic microtubule behavior. Nature (Lond.). 272:450-452. 24, Mcln~sh, J. R., P. K. Helpler, and D. G. Van Wie. 1969. Model for mitosis. Nature (Lond.). 224:659-663. 25. Murphy, D. B., and L. G. Tilney. 1974. The role of microtubules in the movement of pigment granules in telenst melanophores. J. Cell Biol. 61:757-779. 26, Raine, C. S., B. Getti, and M. L. Shelauski. 1971. On the association between microtubules and mitochondria within axons. Brain Res. 34:389-393. 27. Rebhun, L. I. 1972. Polarized intracellular transport: saltatory movements and cytoplasmic streaming. Int. Rev. CytoL 32:92-137. 28. Schliwa, M. S., J. van Blerkom, and K. R. Porter. 1981. Stabilization of the cytoplasmic ground substance in detcrgent-opaned ceils and a structural and a biochemical analysis of its composition. Proc. Natl. Acad. Sci. USA. 73:4329-4333. 29. Smith, D. S., U. Jarifors, and B. F. Cameron. 1975. Morphological evidence for the participation of microtubules in axonal transport. Ann. N. E Acad. Sci. 253:472-506. 30. Smith, D. S., U. Jarlfors, and M. L. Cager. 1977. Structural ~ b r i d g e s between microtubules and mitocbondria in central axons of an insect (Periplaneta americana). J. Cell Sci. 27:235-272. 31. Stearns, M. E., and D. P. Bnggs. 198 I. Studies of axonal transport in digitonin permeated neuroblastoma cells. J. Cell Biol. 91(2, Pt. 2):420a. (Abstr.) 32. Telzer, B. R., and L. T. Haimo. 1981. Decoration of spindle microtubules with dynein: evidence for uniform polarity. J. Cell Biol. 89:373-378. 33. Travis, J. L. 1981. Motility of the reticulopodial network of Allogromia laticollaris. Ph.D. thesis. Dartmouth College, Hanover, NH. 168 pp. 34. Travis, J. L, and R. D. Allen. 1981. Studies on the motility of the foraminifera. I. URrastructure of the reticulopodial network of Allogromia latticollaris (Arnold). J. Cell Biol. 90:211-221. 35. Travis, J. L., J. F. X. Kenealy, and R. D. Allen. 1983. Studies on the motility of the foraminifera. IL The dynamic mierotubular cytoskelcton of the reticulopodial network of Allogromia laticollaris. J. Cell Biol. 97:1668-1676. 36. Wang, E., and R. D. Goldman. 1978. Functions of cytoplasmic fibers in intracellular movements in BHK-21 cells. J. Cell Biol. 79:708-726. 37. Warner, F. D., and D. R. Mitchell. 1981. Polarity of dynein-microtubule interaction in vitro: cross-bridging between parallel and antiparallel microtubules. J. Cell Biol. 89:3544. HAYDEN AND ALLEN Particle Transport along Single Microtobules 1793