* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Pitfalls in the Diagnosis of Celiac Disease
Periodontal disease wikipedia , lookup
Crohn's disease wikipedia , lookup
Ulcerative colitis wikipedia , lookup
Human leukocyte antigen wikipedia , lookup
Childhood immunizations in the United States wikipedia , lookup
Chagas disease wikipedia , lookup
Kawasaki disease wikipedia , lookup
Schistosomiasis wikipedia , lookup
Inflammatory bowel disease wikipedia , lookup
Autoimmunity wikipedia , lookup
Rheumatoid arthritis wikipedia , lookup
Ankylosing spondylitis wikipedia , lookup
Germ theory of disease wikipedia , lookup
Behçet's disease wikipedia , lookup
Neuromyelitis optica wikipedia , lookup
Globalization and disease wikipedia , lookup
Sjögren syndrome wikipedia , lookup
IgA nephropathy wikipedia , lookup
Coeliac disease wikipedia , lookup
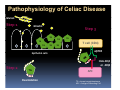
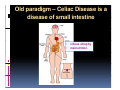
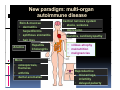
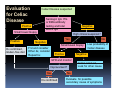
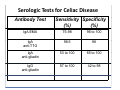
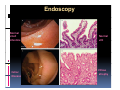
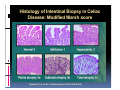
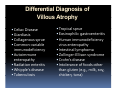
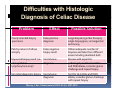
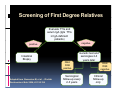
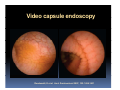
PITFALLS IN THE DIAGNOSIS OF CELIAC DISEASE S. Devi Rampertab, MD Assistant Professor of Medicine Penn State College of Medicine Hershey Medical Center The instructor has no conflicts or professional relationships to disclose. Celiac Disease: The Basics Other names: • Gluten-sensitive enteropathy • Celiac sprue • Non-tropical sprue Chronic autoimmune intestinal disorder Inherited Atrophy of small intestinal villi from chronic iinflammation fl ti resulting lti iin malabsorption l b ti off key nutrients Key inciting trigger is gluten Why is gluten intolerance important? Chronic inflammation from gluten intolerance leads to: Decreased absorption of macro- and micronutrients ti t Increased net secretion of water and solute Involvement In ol ement of m multiple ltiple organ ssystems stems Increased risk of certain malignancies Celiac disease: Epidemiology Once thought to be rare (prevalence was felt to be as low as 1:2000 in many parts of the world) Advent Ad t off serology l h has resulted lt d iin iincreased d recognition of disease Now prevalence of approximately 0.5 to 1 percent in the United States (1 in every 100-200 persons)1 Celiac disease has also been noted worldwide2 1 Fasano 2 A, et al Arch Int Med 2003; 163: 286-292 West J, et al 2003, Maki M, et al 2003, Fasano A,et al 2003, Bingley PJ, et al 2004, Tommasini A, et al 2004 Pathophysiology of Celiac Disease Gluten Innate and Adaptive Immunityy Genetics Celiac C li di disease iis an immune i disorder di d triggered ti db by th the environment (gluten) in genetically susceptible individuals. Pathophysiology of Celiac Disease Gluten Step 1 Gliadin St Step 3 TG TG TG TG TG TG TG T cell (CD4) αβTCR β Epithelial cells h l l ll Step 2 HLA‐DQ2 or ‐DQ8 TG Deamidation APC TG = tissue transglutaminase APC = Antigen Presenting Cell Logan RF, Acta Paediatra Suppl (1996) 412:15-19. Wide Spectrum of Celiac Disease Silent Celiac Disease Latent L t t Celiac C li Disease Dermatitis Herpetiformis Positive P iti bl blood d tests Positive biopsy Negative clinical symptoms Positive blood tests Negative biopsy Negative clinical symptoms Skin manifestation 90% with no GI symptoms Old paradigm – Celiac Disease is a disease of small intestine • • villous atrophy malnutrition New paradigm: multi-organ autoimmune disease Skin & mucosa • dermatitis herpetiformis • aphthous stomatitis • hair loss Anemia Hepatitis Cholangitis Bone • osteoporosis, fractures • arthritis • dental anomalies Central nervous system • ataxia, seizures • depression Carditis, cardiomyopathy • • • villous atrophy malnutrition malignancies Reproductive • miscarriage, miscarriage infertility • delayed puberty Higher Risk Groups E d Endocrine i • Type I Diabetes • Autoimmmune thyroid disorders H Hepatic ti • Primary Biliary Cirrhosis • Autoimmune Hepatitis • Autoimmune Cholangitis Oh Other • First Degree Relatives • Anemia (Iron Deficiency) • Osteoporosis • IgA deficiency • Sjogren’s • Turner Syndome • Down’s syndrome • Infertility Evaluation for Celiac Disease Celiac Disease suspected Serologic IgA tTG or EMA antibody testing and total serum IgA I A Positive Small bowel biopsy Positive Negative High clinical suspicion? Yes es No o Negative Small bowel biopsy Dx confirmed, Gl t f Gluten-free diet di t F/U and consider Other dx, dx consider Repeat bx Positive Low probability of Celiac disease Negative GFD and monitor Improvement? Yes Dx confirmed Celiac ruled out, Look for other cause No Evaluate for possible secondary cause of symptoms Serological Testing Indications: • Abdominal complaints • Malabsorptive symptoms or lab parameters • Hi High h risk i k groups First degree relative of celiac patient Autoimmune illness Other associated conditions Serological Testing Types: T Antigliadin antibodies (IgG and IgA derived) Antireticulin antibodies (IgA derived connective A i i li ib di (I A d i d i tissue antibodies) Antiendomysial (IgA derived connective tissue antibodies) Antibodies against tissue transglutaminase (TTG) t bod es aga st t ssue t a sg uta ase ( G) (IgA derived) Serologic Tests for Celiac Disease Antibody Test Sensitivity Specificity (%) (%) IgA EMA 75-98 96 to 100 IgA anti-TTG 98.5 98 IIgA A anti-gliadin 53 tto 100 65 tto 100 IgG anti-gliadin 57 to 100 42 to 98 Deamidated Gliadin Peptide A tib d Antibody Binding of gliadin to HLA DQ2 or DQ8 is strongly enhanced by deamidation, therefore gy y , there is a greatly enhanced T cell response Influx of studies showing utility of deamidated gliadin peptide as a screening tool for celiac disease Metanalysis: TTG still outperforms g p p deamidated gliadin peptides Deamidated Gliadin Peptide Antibody Tissue Transglutaminase Deamidated Gliadin Peptide Pooled y sensitivity 93.0% 87.8% Pooled Specificity 96.5% 94.1% Endoscopy Normal small intestine Celiac Di Disease Normal villi Villous p y atrophy Histology of Intestinal Biopsy in Celiac Disease: Modified Marsh score Rostami K, et al. Am J Gastroenterol 1999;94:888–894. Differential Diagnosis of Villous Atrophy Celiac Disease C li Di Giardiasis Collagenous sprue Common‐variable immunodeficiencyy Autoimmune enteropathy Radiation enteritis d Whipple’s disease Tuberculosis Tropical sprue T i l Eosinophilic gastroenteritis Human immunodeficiency virus enteropathy Intestinal lymphoma y p Zollinger‐Ellison syndrome Crohn’s disease Intolerance of foods other I l f f d h than gluten (e.g., milk, soy, chicken tuna) chicken, tuna) Difficulties with Histologic Diagnosis of Celiac Disease P bl Problem Eff t Effect P ibl S l ti Possible Solution Poorly oriented biopsy specimens False positive d diagnosis Large biopsies (jumbo forceps); single biopsy/pass; no tangential l b l sectioning Patchy nature of villous atrophy False negative biopsy result Obtain adequate number of biopsies and take from different areas including duodenal bulb Equivocal biopsy result (ie: Inconclusive intraepithelial diagnosis lymphocytosis only)* Review with expert GI pathologist; test for HLA DQ2 and DQ8 alleles; consider gluten challenge and repeat biopsy No initial diagnostic biopsy g p y Inconclusive diagnosis Test for HLA DQ2 and DQ8 alleles; consider gluten challenge with repeat biopsy *Murray, J Am J Gastroenterol 2003; 98 (9): 2027-2033. Diagnosis of Patients Already on a Gl t F Gluten-Free Diet Di t Gluten challenge HLA DQ Testing • At least 2-4 slices of • Does not depend on bread (10-20 g gluten) per day • A 2-month challenge with 8 slices bread ((30 g gluten): converted Marsh 1 (only intraepithelial lymphocytosis) y p ocy os s) to o Marsh as 2 or 3 (crypt hyperplasia alone or with villous atrophy)1 gluten exposure • Only helps if both alleles are negative: virtually excludes the disease (95 % of celiac patients are HLA DQ2 +; rest are HLA DQ8+). Q8 ) • 30% in the general Caucasion population is DQ 2 positive 1Wahab PJ, et al . Am J Gastroenterol 2001;96:1464-1469 HLA Testing: Useful Adjunct to Diagnosis of Celiac Disease Presence can be used to determine which first degree family members need to be screened for celiac disese Useful for ruling out disease in patients already on a gluten‐free diet Can act as another important diagnostic piece in patients whom the diagnosis is unclear Screening of First Degree Relatives Evaluate E l t TTG and d serum IgA (IgG TTG in IgA deficient p patients) ) negative positive Genetic test and serologies 2-3 years later Intestinal Biopsy DQ2/ DQ8 positive Adapted from Bonamico M, et al. J Pediatr Gastroenterol Nutr 2006; 42:150-154 Serological S l i l follow-up every 2-3 years DQ2/ DQ8 negative Clinical Cli i l follow-up only Video capsule endoscopy Rondonotti, E et al. Am J Gastroenterol 2007; 102: 1624-1631 Summary Prevalence of celiac disease has increased due to P l f li di h i d d t increased recognition and advent of serology Current standards for the diagnosis of celiac disease require small bowel histology and improvement on strict gluten gluten-free free diet diet. Serology and genetic tests are supportive, but alone are not sufficient to diagnose celiac disease.