* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Multidrug resistance mediated by the ATP-binding
Survey
Document related concepts
G protein–coupled receptor wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Protein structure prediction wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Signal transduction wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Endomembrane system wikipedia , lookup
Protein moonlighting wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Magnesium transporter wikipedia , lookup
Western blot wikipedia , lookup
Transcript
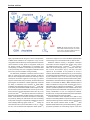
Review articles Multidrug resistance mediated by the ATP-binding cassette transporter protein MRP Susan P.C. Cole* and Roger G. Deeley Summary Resistance to multiple natural product drugs associated with reduced drug accumulation in human tumor cells may be conferred by either the 170 kDa P-glycoprotein or the 190 kDa multidrug resistance protein, MRP. Both MRP and P-glycoprotein belong to the large and ancient ATP-binding cassette (ABC) superfamily of transport proteins but share only 15% amino acid identity. Unlike P-glycoprotein, MRP actively transports conjugated organic anions such as the cysteinyl leukotriene C4 and glutathione-conjugated aflatoxin B1. Transport of unconjugated chemotherapeutic agents appears to require cotransport of glutathione. MRP and several more recently discovered ABC proteins contain an additional NH2-proximal membrane-spanning domain not found in previously characterized ABC transporters. This domain, whose NH2-terminus is extracytosolic, is essential for MRP-mediated transport activity. This review summarizes current knowledge of the structural and transport characteristics of MRP which suggest that the physiologic functions of this protein could range from a protective role in chemical toxicity and oxidative stress to mediation of inflammatory responses involving cysteinyl leukotrienes. BioEssays 20: 931–940, 1998. r 1998 John Wiley & Sons, Inc. Introduction The development of drug resistance is a frequent impediment to the effective treatment of infectious and malignant diseases. Many different resistance mechanisms have been described, but those that involve proteins belonging to the ABC transporter superfamily have been of particular interest because of the increasingly prominent role these proteins play in such devastating and widespread diseases as ma- Cancer Research Laboratories, Queen’s University, Kingston, Ontario, Canada. Funding agencies: Medical Research Council of Canada; National Cancer Institute of Canada; Cancer Care Ontario. *Correspondence to: S.P.C. Cole, Cancer Research Laboratories, Queen’s University, Kingston, Ontario, Canada K7L 3N6. E-mail: [email protected] Abbreviations: MRP, multidrug resistance protein; ABC, ATP-binding cassette; CFTR, cystic fibrosis conductance regulator; MOAT, multispecific organic anion transporter; MSD, membrane-spanning domain; NBD, nucleotide-binding domain; SUR, sulfonylurea receptor; LTC4, leukotriene C4; GSH, glutathione; GSSG, glutathione disulfide; BSO, buthionine sulfoximine; cAMP, cyclic adenosine monophosphate. BioEssays 20:931–940, r 1998 John Wiley & Sons, Inc. laria, leishmaniasis, and cancer. Several mammalian ABC proteins have attracted further attention because of their involvement in essential physiological processes. Important examples of such proteins include the sulfonylurea receptor SUR1, a subunit of the ATP-sensitive K⫹ channel present in the plasma membrane of pancreatic  cells involved in modulating insulin secretion;(1) CFTR, a cAMP-regulated chloride channel;(2) and the transporters associated with antigen presentation TAP1 and TAP2, proteins essential for the transport of peptides in association with major histocompatibility class I molecules.(3) Mutations in several ABC genes are also now known to be the underlying cause of a variety of human genetic disorders. For example, persistent hyperinsulinemia hypoglycemia of infancy is associated with mutations in SUR1;(4) congenital hyperbilirubinemia (Dubin-Johnson syndrome) is associated with mutations in the gene encoding MOAT;(5) cystic fibrosis is caused by mutations in CFTR; (6) and recently, the RIM or ABCR protein in the outer segments of rod photoreceptor cells has been identified as the protein affected in Stargardt’s disease, characterized by a progressive loss of central vision in children.(7,8) Structural features common to all known ABC transporter BioEssays 20.11 931 Review articles Figure 1. Chemical structures of selected clinically useful natural product drugs to which MRP and P-glycoprotein confer resistance. proteins include a hydrophobic polytopic MSD with up to six transmembrane helices and a hydrophilic cytosolic NBD containing three highly conserved sequence motifs, viz., the Walker A and B motifs and the so-called active transport family signature.(9,10) In bacterial ABC proteins, these structural domains are usually encoded as separate polypeptides, which then associate with one another to form an active transporter. Eukaryotic ABC transporters, on the other hand, frequently contain four domains configured either as two alternating MSDs and NBDs encoded as a single large polypeptide (e.g., CFTR) or as two independently encoded ‘‘half-molecules’’ which then associate to function as a heterodimer (e.g., TAP1/TAP2). Presently, more than 200 prokaryotic and eukaryotic ABC proteins have been cloned and characterized to differing degrees. As sequence databases expand with increasing rapidity, it seems likely that the roster of ABC transporters will follow suit.(11) Drug resistance in human cancer Drugs belonging to many different chemical classes are used in the treatment of human tumors, and some of the most powerful of these are the so-called natural product drugs. As their name implies, these drugs were originally isolated from plants, bacteria, or fungi. Semisynthetic or synthetic derivatives of these naturally occurring toxins, which are typically 932 BioEssays 20.11 complex multiheterocyclic structures, have become the mainstay of many commonly used and effective therapeutic protocols. Key among them are the anthracyclines (e.g., doxorubicin and daunorubicin), the Vinca alkaloids (e.g., vincristine and vinblastine), the epipodophyllotoxins (e.g., VP-16 and VM-26), and the taxanes (e.g., paclitaxel) (Fig. 1). These drugs exert their lethal effects by interacting with multiple targets within the tumor cell, and to maximize cytotoxicity, they are almost always administered to patients in combination with other drugs (e.g., antimetabolites, alkylating agents, etc.). The first ABC protein demonstrated to confer resistance to multiple natural product drugs used in the treatment of cancer was the 170 kDa P-glycoprotein, originally described in 1976.(12,13) In humans, this protein is encoded by the MDR1 gene and in mice by the mdr1a (also called mdr1) and mdr1b (also called mdr3) genes. For more than 10 years, these so-called type I isoforms of P-glycoprotein were widely believed to be the only proteins capable of conferring multidrug resistance in mammalian cells. However, several reports of human tumor cell lines displaying multidrug resistance in the absence of P-glycoprotein overexpression(14–16) together with studies that failed to detect P-glycoprotein in a variety of human tumors,(17) pointed to the existence of other multidrug resistance-conferring proteins. The cloning in 1992 Review articles Figure 2. Domain organization of MRP and its related proteins. The figure illustrates two models of membrane topologies of MRP and its related proteins, both of which contain three membrane-spanning domains (MSD1, MSD2, and MSD3) and two nucleotide binding domains (NBD1 and NBD2). A multiple sequence alignment of human and mouse MRP/mrp, human and rabbit MOAT/EBCR, and YCF1 was used as input for profile-based neural network topology prediction by the PredictProtein server.(82) The model shown in A is the most compatible with the neural network output and consists of five transmembrane helices in MSD1, six in MSD2, and four in MSD3. This topology correctly places the NH2-terminus on the extracytoplasmic side of the membrane. The model of MSD3 in B shows a more conventional ABC transporter configuration of six transmembrane segments. of the cDNA encoding the 190 kDa MRP from a doxorubicinselected lung cancer cell line followed shortly thereafter by MRP cDNA transfection experiments provided unequivocal evidence that overexpression of a second ABC protein could cause multidrug resistance in mammalian cells.(18–20) Multidrug resistance conferred by P-glycoprotein and MRP is characterized by an ATP-dependent reduction in drug accumulation. However, despite this common ability to mediate multidrug resistance, there is now a preponderance of evidence demonstrating that these two mammalian ABC proteins differ substantially with respect to a number of important structural, mechanistic, and pharmacological properties. MRP and its relationship to the ABC superfamily On the basis of its deduced amino acid sequence, MRP was predicted to be an integral membrane ATP-binding, N-glycoyslated phosphoprotein of 1,531 amino acids with a polypeptide molecular weight of 171 kDa.(18,21) Studies in both drugselected and MRP-transfected cells from several laboratories have provided supporting experimental evidence demonstrating that MRP does indeed possess many of the biochemical features predicted by its primary structure.(22,23) In addition, it has become apparent that MRP has several structural features that distinguish it from previously known ABC proteins. Prior to the cloning of MRP, ABC proteins were believed to contain a maximum of two MSDs. The presence of a third NH2-proximal hydrophobic region was first noted in MRP(18) and subsequently observed in several other more recently characterized ABC proteins (Fig. 2). All of the proteins that contain a third MSD are more closely related to MRP and to each other than they are to any other member of the ABC superfamily.(24,25) In addition to the mammalian SUR and MOAT proteins, these MRP-related transporters have been identified in a variety of species, such as the heavy metal resistance protein MRP1 in Caenorhabditis elegans,(26) the cadmium resistance factor protein YCF1 and oligomycin resistance protein YOR1/YRS1 in Saccharomyces cerevisiae,(27,28) and the GSH conjugate transporter AtMRP1 from the plant Arabidopsis thaliana.(29) Thus, there are now at least a dozen ABC proteins with a third NH2-terminal MSD which together comprise the MRP branch of the superfamily (Table 1). The amino acid similarity/identity of these proteins to MRP is relatively high and ranges from 50%/30% (Yor1/Yrs1) to 67%/49% (human MOAT). In contrast, these MRP-related transporters all share less than 20% amino acid identity with the P-glycoproteins. Comparison of the primary structures of MRP-related proteins and, where available, the intron/exon organization of their respective genes suggests that they may have evolved by fusion of a gene encoding a common, four-domain ancestor with a gene encoding the additional NH2-terminal MSD.(30,31) Similar comparisons of members of the MRP branch of the superfamily with ABC proteins containing four domains indicate that they are most likely to have shared this ancestor with CFTR. It is difficult to predict whether or not there was in fact a common five-domain ancestor for the MRP-related proteins because the primary structures of the NH2-terminal MSDs are considerably more divergent than the remainder of the proteins.(29) Consequently, the MRP branch may have evolved as the result of several independent gene fusion events. Alternatively, functional constraints on the BioEssays 20.11 933 Review articles TABLE 1. Similarity Analyses and Functions of Human MRP and its Most Closely Related ABC Proteins Protein/gene (species) Function % Identity % Similarity MRP (human) mrp (mouse) MOAT (human)/MRP2 EBCR (rabbit) C. elegans mrpl (nematode) C. elegans mrp2 (nematode) MRP6 (human) YCF1 (yeast) AtMRP1 (Arabidopsis) SUR1 (human) sur2 (rat, mouse) YOR1/YRS1 (yeast) LtpgpA (leishmania) GS-X pump, anionic conjugate transporter, multidrug resistance GS-X pump, anionic conjugate transporter, multidrug resistance GS-X pump, anionic conjugate transporter (hepatocanaliculi) Probable MOAT ortholog Heavy metal resistance Unknown Unknown Cadmium resistance, vacuolar GS-X pump GS-X conjugate pump Sulfonylurea receptor, K⫹ channel regulator (pancreas) Sulfonylurea receptor, K⫹ channel regulator (brain, heart) Oligomycin resistance Resistance to antimonial and arsenical oxyanions 100 89.9 48.7 48.7 46.3 46.6 42.1 40.2 36.0 33.1 32.5 30.3 30.0 100 96.1 66.9 66.6 64.2 63.6 60.6 59.9 55.0 53.2 53.1 50.0 47.9 Sequences were aligned along their entire length with MRP using CLUSTAL W(1.6) multiple sequence alignment. Sequence data were obtained using the following accession numbers: MRP, L05628/P33527; mrp, AF022908/1488428; MOAT, U49248/U63970; EBCR, 1430907/Z49144; C. elegans mrp1, U66260; C. elegans mrp2, U66261; MRP6, U91318; YCF1, L35327/Z48179; AtMRP1, AF008124; SUR1, L78207/U63421; sur2, D83598/D86037; YOR1/YRS1, Z73066; LtpgpA, X17154. Several additional MRP-related proteins were not included because their complete cDNA sequences have not yet been published. primary structure of the additional MSDs may be low compared with other regions of the proteins. As we learn more about the structure/function relationships of the third MSD, it may be possible to make a more educated guess. A second distinctive structural feature of MRP pertains to its NBDs. The similarity between the two NBDs of the P-glycoproteins is quite high, and although cooperativity between these domains is required for activity,(32) there is no evidence that they are functionally distinct in any significant way. In contrast, the two NBDs of MRP (which are also both required for function)(33) and its related proteins, as well as CFTR, are considerably less similar to each other. Furthermore, there is convincing experimental evidence indicating that NBD1 and NBD2 of CFTR are functionally distinct, with NBD1 believed to be important in Cl– channel opening and NBD2 important in the prolongation of the open channel state and channel closing.(34) Whether or not the two NBDs of MRP are also functionally distinct remains to be determined. There are 11 amino acids present in the NBDs of the P-glycoproteins that are absent from the comparable location in NBD1 of most of the MRP-related proteins, which significantly shortens the distance between the Walker A and B motifs. This alternate spacing of the Walker A and B motifs is also present in NBD1 of CFTR and is one of the shared features suggesting evolution of MRP and CFTR from a common ancestor.(30,31) The distance between the Walker A and B motifs in NBD1 of SUR is altered in a different manner from that for the other MRP-related transporters, in that there are 14 more amino acids (rather than 11 less) at this location when compared to the P-glycoproteins. Whether or not this particular spacing has consequences on ATP binding and 934 BioEssays 20.11 hydrolysis as they relate to SUR function remains to be elucidated. What appears common among all ABC transporters characterized to date, including MRP,(33) is that the conserved lysine residue in the Walker A motif (GX4GKS/T) in both NBDs is essential for full transport function, consistent with the role of this consensus sequence as the amino acid acceptor site of the phosphoryl moiety of the nucleotide. Also of possible significance with respect to the binding and hydrolysis of nucleotide is the observation that the ABC ‘‘active transport family’’ signature (LSSGGQX3RHydXHydA), which is relatively invariant in both NBD1 and NBD2 of the P-glycoproteins, is highly conserved in NBD1, but not in NBD2, of the MRP-related proteins. Mutational studies indicate that this sequence acts as more than just a linker between the Walker A and B motifs in CFTR,(35) and by analogy, it seems probable that this motif will also have a functional role in MRP.(33) Thus, the distinctive structural features of NBD1 and NBD2 of MRP and its related proteins are highly likely to have important consequences with respect to the mechanism of ATP binding and hydrolysis as well as the presumed coupling of hydrolysis to transport of substrate.(36) Transport functions of MRP As mentioned previously, drug resistance conferred by MRP and the P-glycoproteins is usually associated with an ATPdependent reduced cellular accumulation of drug. It has been established by several laboratories that human MRPtransfected mammalian cells, like P-glycoprotein-transfected cells, are resistant to vincristine, doxorubicin, daunomycin, and VP-16 and accumulate lower steady-state levels of these Review articles drugs than do control transfected cells.(20,33,37) Interestingly, rather lower levels of resistance to vinblastine, colchicine, and paclitaxel are observed in MRP transfectants than in P-glycoprotein-overexpressing cells, but the basis of this differential sensitivity is unknown. Also intriguing, but presently unexplained, is the lack of anthracycline resistance in cells transfected with the murine ortholog of MRP.(38) Finally, in addition to chemotherapeutic agents, MRP also confers resistance to certain antimonial and arsenical oxyanions, a property not associated with overexpression of P-glycoprotein.(37,38) Thus, although the drug-resistance profiles of these two drug-resistance proteins are similar, they are not identical. It has long been noted in cells that overexpress P-glycoprotein that the degree of reduced drug accumulation seldom correlates well with the relative resistance of the cells. This is also true of cells that overexpress MRP, and a generally satisfactory explanation for these observations remains elusive. Enhanced drug efflux is usually easily demonstrable in P-glycoprotein-overexpressing cells, whereas in MRP-overexpressing cells, enhanced drug efflux is less pronounced, even in cells that display relatively high levels of resistance (50- to 100-fold) and express substantial amounts of the protein. Interpretation of these observations is difficult, and it is widely agreed that, in most cases, drug transport studies and kinetic analyses in intact cells are fraught with experimental complications that require a number of assumptions to be made, the validity of which cannot be readily established. To avoid some of these problems, the transport properties of P-glycoprotein and MRP have also been studied more directly in inside-out membrane vesicle systems. This experimental approach utilizes a rapid filtration technique to measure the uptake of radiolabeled substrate into vesicles and allows for more rigorous analyses of kinetic parameters. In contrast to studies of membrane vesicles enriched for P-glycoprotein,(39,40) it has not been possible to demonstrate MRP-mediated active transport of chemotherapeutic drugs such as vincristine, daunorubicin, and VP-16,(38,41–43) and contrary reports claiming to have shown direct transport of these compounds have recently been retracted.(44) Consistent with the lack of direct transport is the inability to label MRP with photoactive radiolabeled analogs of vinblastine or doxorubicin.(37,45) A possible explanation for the inability of MRP to bind and transport these chemotherapeutic agents, and a clue to one of the protein’s potential physiological roles, was provided by the demonstration that MRP in inside-out membrane vesicles can function as a high-affinity, primary active transporter of the cysteinyl leukotriene, LTC4.(42,46) MRP can also actively transport a variety of other GSH-conjugated xenobiotics, including the GSH conjugates of the activated forms of the potent carcinogen aflatoxin B1 (Fig. 3).(47) These latter findings suggest that MRP may serve a protective role in chemical carcinogenesis. Several other proteins of the MRP branch of the superfamily, such as MOAT, YCF-1, and Figure 3. Chemical structures of examples of anionic conjugated xenobiotics and endobiotics actively transported by MRP. AtMRP1, are also known to transport GSH conjugates in vitro,(29,48,49) indicating that at least some of these proteins share a similar substrate specificity distinct from that of the P-glycoproteins and other ABC transporters. How then to explain the inability of MRP to transport the drugs to which it confers resistance? Consideration of the molecular structure of LTC4 prompted the suggestion that MRP transported anionic conjugates of the drugs rather than the unmodified drugs themselves. However, there are several reasons why this is an unlikely general explanation for the ability of the protein to confer resistance to such a structurally diverse spectrum of antineoplastic agents in such a wide variety of cell types. First, and most compelling, is the well-established observation that, with few exceptions, phase II conjugation plays a relatively minor role in the in vivo and in vitro metabolism of these compounds. In addition, phase II biotransformation reactions are known to occur primarily in the liver (and to a much lesser extent in other tissues) and it is BioEssays 20.11 935 Review articles Figure 4. Models depicting ATP-dependent, MRP-mediated transport of leukotriene C4 and co-transport of GSH and vincristine across the plasma membrane. highly improbable that all cell types in which overexpression of MRP causes resistance are competent to carry out such conjugations with the efficiency and completeness required to cause resistance. Finally, phase II conjugation usually (but not always) results in detoxification of xenobiotics, and consequently, it is difficult to rationalize why enhanced efflux of the presumably less toxic conjugated metabolites would have such a pronounced effect on drug sensitivity. The alternative possibilities considered were that either efflux of certain drugs might require activation of MRP by GSH (or other endogenous small anionic molecule) or else a cotransport mechanism might be involved (Fig. 4). Indirect support of these ideas was provided by the observation that physiological concentrations of GSH significantly enhanced the ability of vinblastine and vincristine to inhibit MRPmediated, ATP-dependent transport of LTC4.(42) It was then more directly demonstrated that MRP could transport unmodified vincristine (and later aflatoxin B1 and VP-16) into membrane vesicles in an ATP-dependent manner but only in the presence of GSH.(42,47) This effect of GSH was concentrationdependent and inhibitable by MRP-specific monoclonal antibodies. It was shown not to be the consequence of activating the protein by altering its redox state since the nonreducing methyl-GSH conjugate could partially substitute for GSH but other sulfhydryl-reducing agents could not.(42,47) Finally, the observation that the converse stimulation occurs (i.e., vincristine and VP-16 stimulate ATP-dependent GSH uptake(83)) 936 BioEssays 20.11 lends further support to the notion that MRP-mediated efflux of these drugs occurs in association with an efflux of GSH. Additional evidence favoring a drug/GSH cotransport mechanism has been obtained from studies using murine mrp double-knockout (mrp–/–) cell lines.(50,83) Thus, export of GSH from murine wild-type (mrp⫹/⫹) embryonic stem cells, but not from the knockout (mrp–/–) cell lines, has been reported to be increased in the presence of VP-16.(50) However, this stimulation of GSH export only occurred at drug concentrations that exceeded the IC50 of VP-16 in these cells by 50- to100-fold, and it is not clear why such high drug concentrations are required to observe this stimulatory effect. Nevertheless, these observations suggest that, since GSH by itself is not a substrate for MRP,(51) MRP-mediated export of GSH in the absence of drug or other xenobiotic may occur in association with an endogenous metabolite(s).(50) This would provide a possible explanation for the reduced levels of GSH previously observed in certain MRP-overexpressing cells.(52,53) Clearly, identification of such an endogenous substrate, should it exist, would provide additional important information about the physiological function(s) of MRP. A further indication of the important role of GSH in drug transport is the ability of BSO to improve the efficacy of some natural product drugs in both cultured cells and mice bearing tumors that express elevated levels of MRP.(52,54,55) BSO depletes cellular GSH levels by irreversibly inhibiting ␥-glutamylcysteine synthetase, the rate-limiting enzyme of the GSH Review articles biosynthetic pathway. Although these observations are intriguing, a general mechansim that explains MRP-mediated resistance remains elusive. This is particularly true with respect to the anthracycline antibiotics since GSH displays little or no ability to enhance either their transport directly or their ability to inhibit ATP-dependent, MRP-mediated transport of LTC4.(42) We have also found that treatment of either drug-selected or transfected cells with BSO restores sensitivity to vincristine far more effectively than to doxorubicin.(52,53) While remaining incompletely understood, these data are consistent with the apparently greater importance of GSH in MRP-mediated transport of Vinca alkaloids compared to anthracyclines. Despite considerable effort by numerous investigators, it is still not clear precisely how P-glycoprotein recognizes such a broad spectrum of structurally diverse hydrophobic substrates.(56) In the case of MRP, and probably its related proteins, there is the additional complication of understanding how substrate selectivity with respect to a cotransport mechanism may occur. Like many other ABC proteins, it is presumed that ATP hydrolysis provides the energy for MRP-mediated transport of substrate (with or without GSH), but precisely how transport is coupled to the binding and hydrolysis of nucleotide is unknown. There is some evidence that MRP-enriched membranes exhibit basal ATPase activity that can be stimulated, albeit modestly, by certain drugs.(57,58) The level of this activity appears substantially lower than the constitutive and drugstimulated ATPase activities typically reported for P-glycoprotein and is more similar to the low levels reported for CFTR.(36,59) This low activity and modest stimulation by substrate make it difficult to distinguish ATPase activity attributable to MRP from other ATPase activities present in the membrane preparations. More definitive studies will require the purification of MRP and its reconstitution into a defined liposomal environment. Purification of mammalian ABC proteins such as P-glycoprotein and CFTR that have retained their activity has proven to be a challenging task, as might be expected for any large integral membrane protein, but a variety of experimental approaches have recently proven successful.(56,60) One group has recently reported that histidine-tagged recombinant MRP purified from transfected hamster cells by Ni2⫹-chelate chromatography exhibited a basal ATPase activity in the presence of sheep brain lipid with an estimated Km of 3 mM and Vmax of 0.46 µmol mg-1 min-1 and that this activity could be stimulated by certain MRP substrates, including (somewhat surprisingly) unmodified drugs.(61) However, the estimated affinity of the purified protein for ATP, although comparable to that for P-glycoprotein, is markedly lower than that obtained from transport assays in which MRP is present in membrane vesicles (Km for ATP approximately 100 µM). Furthermore, stimulation of the ATPase activity of the solubilized protein by LTC4 (previously reported in several different membrane vesicle systems to exhibit a Km of approximately 50–100 nM) showed no dosedependent increase over a 1,000-fold concentration range (1 nM–1 µM). However, MRP purification and reconstitution studies are at an early stage, and it is likely that these apparently discordant findings will be resolved as we learn more about the influence of the membrane environment on MRP topology and function. Physiological functions of MRP In addition to LTC4, it has been shown that other endogenously formed organic anion conjugates previously proposed as putative physiological substrates of P-glycoprotein, such as 17-estradiol 17-(-D-glucuronide), bilirubin glucuronides, and some sulfated bile salts, are actively transported by MRP in vitro.(43,62,63) However, although MRP may be an efficient transporter of certain glucuronides, glucuronate itself, unlike GSH, does not stimulate the active transport of unmodified xeno- or endobiotics. Another endobiotic shown to be actively transported by MRP in vitro is oxidized GSH or GSSG.(51) The identification of this compound as a potential physiological substrate raises the possibility of a role for MRP in cellular defenses against oxidative stress and perhaps also the maintenance of intracellular redox potential. As mentioned previously, the protein with greatest sequence similarity to MRP is MOAT (sometimes referred to as MRP2), and these two proteins share a very comparable substrate specificity.(49) There are some data to suggest that MOAT, like MRP, is capable of drug transport,(64) and it may also be capable of conferring resistance, at least in some cell types, since enhanced sensitivity to certain drugs (i.e., cisplatin, vincristine, and doxorubicin but not VP-16) has been observed in cells transfected with a MOAT antisense cDNA.(65) On the other hand, the tissue distribution and subcellular localization of MRP and MOAT are strikingly different, implying different physiological functions. Thus, levels of MRP are highest in the lung, testis, and muscle and very low in liver,(18,24,66) whereas MOAT levels are highest in liver and relatively lower in other tissues.(67) Furthermore, in rat and human liver, MOAT is predominantly in canalicular (apical) membranes, while the small amounts of MRP in this tissue are predominantly on basolateral membranes.(68) MRP has also been shown to localize to basolateral membranes when transfected into polarized kidney cells.(64) What structural features of these two proteins contribute to their different subcellular locations are presently unknown. Two groups of investigators have recently independently reported the generation and preliminary characterization of MRP-deficient mrp( –/–) mice using embryonic stem cell technology.(69,70) In both cases, the mice were viable and fertile, indicating that MRP is dispensable for development in these animals, as was previously demonstrated for the P-glycoproteins (mdr1a, mdr1b, and mdr2).(71) In the one study, the response of the mrp knockout mice to an inflamma- BioEssays 20.11 937 Review articles tory stimulus was impaired. This was attributed to decreased LTC4 secretion, which was demonstrated in bone marrowderived mast cells obtained from the mice, thus supporting a physiological role for MRP as a transporter of cysteinyl leukotrienes.(69) These same mice were also hypersensitive to VP-16 but not vincristine, although their mast cells displayed increased sensitivity to both drugs when cultured in vitro. In the second study,(70) the mrp( –/–) mice were also found to be hypersensitive to VP-16 but vincristine sensitivity and sensitivity to an inflammatory stimulus were not tested. Consequently, it is not known whether the phenotypes of these independently derived knockout mice are identical. Further characterization of mrp( –/–) mice should continue to be enlightening with respect to the physiological function(s) of MRP, its potential protective role in oxidative stress and chemical toxicity, and the mechanism by which it affects drug sensitivity. MRP topology and higher-order structure To fully understand how the binding of substrates and hydrolysis of ATP by MRP (or other ABC transporter) are coupled to transport will require knowledge of the membrane topology and higher-order structure of the protein. These types of investigations are greatly aided by recent and ongoing developments in computer-assisted hydropathy and modeling methods to predict structures that can then be subjected to experimental verification. One approach that has been useful for distinguishing among several possible topological models of MRP has been to use various antibodies against known regions of the protein combined with limited proteolysis studies to map the location of specific peptide sequences on one side of the plasma membrane or the other. Another has been to use site-directed mutagenesis of potential N-glycosylation sites to determine which ones are actually used. Thus, by mapping the cytoplasmic epitope of the MRP-specific monoclonal antibody QCRL-1 to amino acids 918–924, it was shown that the linker region connecting the two ‘‘halves’’ of the molecule is indeed cytosolic, as expected, and, moreover, that this region is hypersensitive to proteolysis.(72,73) Furthermore, immunostaining of permeabilized cells, but not intact cells, with antisera and monoclonal antibodies recognizing NBD1 and NBD2 confirmed the cytoplasmic location of these hydrophilic regions. Similarly, the sequence linking MSD1 to MSD2 has been localized to the cytoplasm using a monoclonal antibody that maps to amino acids 238–247 and the COOH-terminus has been confirmed to be cytoplasmic with a monoclonal antibody mapping to amino acids 1511–1520.(22,74,75) Finally, by determining that Asn19 and Asn23 are the only sites of N-linked glycosylation in the NH2-terminal MSD1, it was revealed that MRP has an extracytosolic NH2-terminus,(76) a finding that has been independently confirmed and extended by epitope insertion studies.(77) These data indicated that 938 BioEssays 20.11 MSD1 of MRP contains an odd number of transmembrane helices, a somewhat unexpected topology for an ABC protein. The extracytosolic location of the NH2-terminus was also unexpected, but since the NH2-termini of murine mrp and rat SUR are also known to be extracytosolic,(78) it may be that this topological feature is shared by all proteins in the MRP subgroup of the ABC superfamily. A complementary approach to understanding the structure/ function relationships of an ABC protein is to express the individual structural domains (and variants thereof) by themselves and in various combinations in a heterologous system. The ability of the coexpressed fragments of the protein to interact with each other can then be measured using a functional assay or demonstrated more directly by coimmunoprecipitation procedures. Studies using a baculovirus system to express MRP (and its component domains) in Sf21 insect cells have been quite informative with respect to our knowledge of the structural requirements for MRP-mediated LTC4 transport. Thus, for example, we have shown that the two ‘‘halves’’ of MRP need not be covalently linked to one another for active transport of LTC4 to occur,(79) and indeed, amino acids 859–931 in the so-called connecting region of the molecule appear to be dispensable for activity.(80) On the other hand, a polypeptide missing the first 280 amino acids of MRP (MRP281–1531) is virtually inactive, indicating that MSD1 is critically important for LTC4 transport function.(80) MSD1 is the most divergent of the five domains of the MRP-related ABC proteins,(29) and while the LTC4 transport activity of MRP281–1531 can be restored by coexpression of MSD1 (MRP1–281), a chimeric protein consisting of the corresponding MSD1 of MOAT fused to MRP281–1531 (MOAT1–280MRP281–1531) is inactive. These data point to a key, and probably essential, role of the first MSD1 in the active transport function(s) of MRP and possibly its related proteins as well. Finally, an approach to investigating the physical interactions of the various domains of MRP that has not yet been explored is by electron microscopy and image analysis. This has recently proven feasible for both detergent-solubilized and lipid-reconstituted P-glycoprotein, for which an initial structure to 2.5 nm resolution has been derived that is consistent with most available biochemical data.(81) Thus, these studies indicate that P-glycoprotein is a monomeric cylindrical protein with an external diameter of about 10 nm and a maximum height of about 8 nm. Traversing this cylinder is a large central pore with a diameter of about 5 nm that is closed at the inner (cytoplasmic) face of the membrane, thus forming an aqueous chamber within the membrane. Further, the surface area occupied by P-glycoprotein in the membrane is estimated to be about 60 nm2 and the 12 transmembrane ␣-helices of the protein thus appear to be loosely packed. Similar investigations of MRP should prove extremely interesting, in view of the significant structural differences between Review articles the two proteins that are already known from biochemical analyses. Conclusions Investigations of the function and structure of MRP as well as its role in drug resistance in clinical oncology have been greatly aided by reference to the established paradigm of P-glycoprotein. Nevertheless, despite their shared ability to confer resistance to multiple natural product antineoplastic drugs, these two proteins are structurally and mechanistically quite distinct and almost certainly have different physiological functions. Unlike P-glycoprotein, MRP has the ability to transport conjugated organic anions and cotransport of GSH appears to be required for the transport of certain xenobiotics. Furthermore, MRP was the first example of a subgroup of the ABC superfamily whose members do not conform to the typical four-domain structure of previously characterized ABC transporters. Thus, in addition to being of major clinical relevance, the cloning and characterization of MRP may be considered a key event in the discovery of a new branch of this large and ancient superfamily of active transporters that has facilitated new avenues of investigation. Acknowledgments We apologize to the many investigators whose work was not cited because of space limitations. We thank the members of our laboratories, both past and present, for their contributions to some of the work described. Special acknowledgment is due to Dr. James H. Gerlach for helpful discussions as well as expert and insightful sequence analyses. We thank Elaine M. Leslie for assistance in compiling the information in Table 1 and Alexander J. Lang for technical assistance with the figures. REFERENCES 1 Bryan J, Aguilar-Bryan L (1997) The ABCs of ATP-sensitive potassium channels: more pieces of the puzzle. Curr Opin Cell Biol 9: 553–559. 2 Riordan JR, Rommens JM, Kerem B-s, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plasvsic N, Chou J-L, Drum ML, Iannuzzi MC, Collins FS, Tsui L-C (1989) Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245: 1066–1073. 3 Kelly A, Powis SH, Kerr L-A, Mockridge I, Elliott T, Bastin J, Uchanska-Ziegler B, Ziegler A, Trowsdale J, Townsend A (1992) Assembly and function of the two ABC transporter proteins encoded in the human major histocompatibility complex. Nature 355: 641–644. 4 Dunne MJ, Kane C, Shepherd RM, Sanchez JA, James RFL, Johnson PRV, Aynsley-Green A, Lu S, Clement JP, Lindley KJ, Seino S, Aguilar-Bryan L (1997) Familial persistent hyperinsulinemic hypoglycemia of infancy and mutations in the sulfonylurea receptor. N Engl J Med 336: 703–706. 5 Kartenbeck J, Leuschner U, Mayer R, Keppler D (1996) Absence of the canalicular isoform of the MRP gene-encoded conjugate export pump from the hepatocytes in Dubin-Johnson syndrome. Hepatology 23: 1061– 1066. 6 Zielenski J, Tsui L-C (1995) Cystic fibrosis: genotypic and phenotypic variations. Annu Rev Genet 29: 777–807. 7 Illing M, Molday LL, Molday RS (1997) The 220-kDa rim protein of retinal rod outer segments is a member of the ABC transporter superfamily. J Biol Chem 272: 10303–10310. 8 Azarian SM, Travis GH (1997) The photoreceptor rim protein is an ABC transporter encoded by the gene for recessive Stargardt’s disease (ABCR). FEBS Lett 409: 247–252. 9 Walker JE, Saraste M, Runswick MJ, Gay NJ (1982) Distantly related sequences in the ␣- and -subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J 1: 945–951. 10 Higgins CF (1992) ABC transporters: from microorganisms to man. Annu Rev Cell Biol 8: 67–113. 11 Allikmets R, Gerrard B, Hutchinson A, Dean M (1996) Characterization of the human ABC superfamily: isolation and mapping of 21 new genes using the Expressed Sequence Tags database. Hum Mol Genet 5: 1649– 1655. 12 Juliano RL, Ling V (1976) A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta 455: 152–162. 13 Gottesman MM, Pastan I, Ambudkar SV (1996) P-glycoprotein and multidrug resistance. Curr Biol 6: 610–617. 14 Mirski SEL, Gerlach JH, Cole SPC (1987) Multidrug resistance in a human small cell lung cancer cell line selected in adriamycin. Cancer Res 47: 2594–2598. 15 McGrath T, Center MS (1987) Adriamycin resistance in HL60 cells in the absence of detectable P-glycoprotein. Biochem Biophys Res Commun 145: 1171–1176. 16 Zijlstra JG, de Vries EGE, Mulder NH (1987) Multifactorial drug resistance in an adriamycin-resistant human small cell lung carcinoma cell line. Cancer Res 47: 1780–1784. 17 Goldstein LJ, Galski H, Fojo A, Willingham M, Lai S-L, Gazdar A, Pirker R, Green A, Crist W, Brodeur GM, Lieber M, Cossman J, Gottesman MM, Pastan I (1989) Expression of a multidrug resistance gene in human cancers. J Natl Cancer Inst 81: 116–124. 18 Cole SPC, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AMV, Deeley RG (1992) Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 258: 1650–1654. 19 Grant CE, Valdimarsson G, Hipfner DR, Almquist KC, Cole SPC, Deeley RG (1994) Overexpression of multidrug resistance-associated protein (MRP) increases resistance to natural product drugs. Cancer Res 54: 357–361. 20 Zaman GJR, Flens MJ, Van Leusden MR, de Haas M, Mulder HS, Lankelma J, Pinedo HM, Scheper RJ, Baas F, Broxterman HJ, Borst P (1994) The human multidrug resistance-associated protein MRP is a plasma membrane drug-efflux pump. Proc Natl Acad Sci USA 91: 8822–8826. 21 Cole SPC, Deeley RG (1993) Multidrug resistance-associated protein: sequence correction. Science 260: 879. 22 Almquist KC, Loe DW, Hipfner DR, Mackie JE, Cole SPC, Deeley RG (1995) Characterization of the 190 kDa multidrug resistance protein (MRP) in drug-selected and transfected human tumor cells. Cancer Res 55: 102–110. 23 Krishnamachary N, Center MS (1993) The MRP gene associated with a non-P-glycoprotein multidrug resistance encodes a 190-kDa membrane bound glycoprotein. Cancer Res 53: 3658–3661. 24 Stride BD, Valdimarsson G, Gerlach JH, Wilson GM, Cole SPC, Deeley RG (1996) Structure and expression of the mRNA encoding the murine multidrug resistance protein (MRP), an ATP-binding cassette transporter. Mol Pharmacol 49: 962–971. 25 Deeley RG, Cole SPC (1997) Function, evolution and structure of MRP. Semin Cancer Biol 8: 193–204. 26 Broeks A, Gerrard B, Allikmets R, Dean M, Plasterk RHA (1996) Homologues of the human multidrug resistance genes MRP and MDR contribute to heavy metal resistance in the soil nematode Caenorhabditis elegans. EMBO J 15: 6132–6143. 27 Szczypka MS, Wemmie JA, Moye-Rowley WS, Thiele DJ (1994) A yeast metal resistance protein similar to human cystic fibrosis transmembrane conductance regulator (CFTR) and multidrug resistance-associated protein. J Biol Chem 269: 22853–22857. 28 Katzmann EJ, Hallstrom TC, Voet M, Wysock W, Golin J, Volckaert G, Moye-Rowley WS (1995) Expression of an ATP-binding cassette transporter-encoding gene (YOR1) is required for oligomycin resistance in Saccharomyces cerevisiae. Mol Cell Biol 15: 6875–6883. 29 Lu Y-p, Li Z-s, Rea PA (1997) AtMRP1 gene of Arabidopsis encodes a glutathione S-conjugate pump: isolation and functional definition of a plant ATP-binding cassette transporter gene. Proc Natl Acad Sci USA 94: 8243– 8248. 30 Grant CE, Kurz EU, Cole SPC, Deeley RG (1997) Analysis of the intron-exon organization of the multidrug resistance protein (MRP) gene and alternative splicing of its mRNA. Genomics 45: 368–378. 31 Tusnady GE, Bakos E, Varadi A, Sarkadi B (1997) Membrane topology distinguishes a subfamily of the ATP-binding cassette (ABC) transporters. FEBS Lett 402: 1–3. 32 Azzaria M, Schurr E, Gros P (1989) Discrete mutations introduced in the predicted nucleotide-binding sites of the mdr1 gene abolish its ability to confer multidrug resistance. Mol Cell Biol 9: 5289–5297. 33 Zhu Q, Sun H, Center MS (1997) Functional analysis of the nucleotide binding domains of the multidrug resistance protein (MRP). Oncol Res 9: 229–236. 34 Carson MR, Travis SM, Welsh MJ (1995) The two nucleotide-binding domains of cystic fibrosis transmembrane conductance regulator (CFTR) have distinct functions in controlling channel activity. J Biol Chem 270: 1711–1717. 35 Carson MR, Welsh MJ (1995) Structural and functional similarities between CFTR and GTP-binding proteins. Biophys J 69: 2443–2448. 36 Senior AE, Gadsby DC (1997) ATP hydrolysis cycles and mechanism in P-glycoprotein and CFTR. Semin Cancer Biol 8: 143–150. BioEssays 20.11 939 Review articles 37 Cole SPC, Sparks KE, Fraser K, Loe DW, Grant CE, Wilson GM, Deeley RG (1994) Pharmacological characterization of multidrug resistant MRP-transfected human tumor cells. Cancer Res 54: 5902–5910. 38 Stride BD, Grant CE, Loe DW, Hipfner DR, Cole SPC, Deeley RG (1997) Pharmacological characterization of the murine and human orthologs of multidrug resistance protein in transfected human embryonic kidney cells. Mol Pharmacol 52: 344–353. 39 Schlemmer SR, Sirotnak FM (1994) Functional studies of P-glycoprotein in inside-out plasma membrane vesicles derived from murine erythroleukemia cells overexpressing MDR3. Properties and kinetics of the interaction of vinblastine with P-glycoprotein and evidence for its active mediated transport. J Biol Chem 269: 31059–31066. 40 Cornwell MM, Pastan I, Gottesman MM (1987) Certain calcium channel blockers bind specifically to multidrug-resistant human KB carcinoma membrane vesicles and inhibit drug binding to P-glycoprotein. J Biol Chem 262: 2166–2170. 41 Muller M, Meijer C, Zaman GJR, Borst P, Scheper RJ, Mulder NH, de Vries EGE, Jansen PLM (1994) Overexpression of the gene encoding the multidrug resistance-associated protein results in increased ATP-dependent glutathione S-conjugate transport. Proc Natl Acad Sci USA 91: 13033–13037. 42 Loe DW, Almquist KC, Deeley RG, Cole SPC (1996) Multidrug resistance protein (MRP)-mediated transport of leukotriene C4 and chemotherapeutic agents in membrane vesicles: demonstration of glutathione-dependent vincristine transport. J Biol Chem 271: 9675–9682. 43 Jedlitschky G, Leier I, Buchholz U, Barnouin K, Kurz G, Keppler D (1996) Transport of glutathione, glucuronate, and sulfate conjugates by the MRP gene-encoded conjugate export pump. Cancer Res 56: 988–994. 44 Kruh GD (1997) Corrections. Biochemistry 36: 13972. 45 McGrath T, Latoud C, Arnold ST, Safa AR, Felsted RL, Center MS (1989) Mechanisms of multidrug resistance in HL60 cells: analysis of resistance associated membrane proteins and levels of mdr gene expression. Biochem Pharmacol 38: 3611–3619. 46 Jedlitschky G, Leier I, Buchholz U, Center M, Keppler D (1994) ATP-dependent transport of glutathione S-conjugates by the multidrug resistance-associated protein. Cancer Res 54: 4833–4836. 47 Loe DW, Stewart RK, Massey TE, Deeley RG, Cole SPC (1997) ATP-dependent transport of aflatoxin B1 and its glutathione conjugates by the product of the MRP gene. Mol Pharmacol 51: 1034–1041. 48 Li Z-s, Szczypka MS, Lu Y-p, Thiele DJ, Rea PA (1996) The yeast cadmium factor protein (YCF1) is a vacuolar glutathione S-conjugate pump. J Biol Chem 271: 6509–6517. 49 Keppler D, Leier I, Jedlitschky G (1997) Transport of glutathione conjugates and glucuronides by the multidrug resistance proteins MRP1 and MRP2. Biol Chem 378: 787–791. 50 Rappa G, Lorico A, Flavell RA, Sartorelli AC (1997) Evidence that the multidrug resistance protein (MRP) functions as a co-transporter of glutathione and natural product toxins. Cancer Res 57: 5232–5237. 51 Leier I, Jedlitschky G, Buchholz U, Center M, Cole SPC, Deeley RG, Keppler D (1996) ATP-dependent glutathione disulfide transport mediated by the MRP gene-encoded conjugate export pump. Biochem J 314: 433–437. 52 Lautier D, Canitrot Y, Deeley RG, Cole SPC (1996) Multidrug resistance mediated by the multidrug resistance protein (MRP) gene. Biochem Pharmacol 52: 967–977. 53 Cole SPC, Downes HF, Mirski SEL, Clements DJ (1990) Alterations in glutathione and glutathione-related enzymes in a multidrug resistant small cell lung cancer cell line. Mol Pharmacol 37: 192–197. 54 Versantvoort CHM, Broxterman HJ, Bagrij T, Scheper RJ, Twentyman PR (1995) Regulation by glutathione of drug transport in multidrug-resistant human lung tumor cell lines overexpressing multidrug resistance-associated protein. Br J Cancer 72: 82–89. 55 Vanhoefer U, Cao S, Minderman H, Toth K, Skenderis BS II, Slovak ML, Rustum YM (1996) D,L-Buthionine-(S,R)-sulfoximine potentiates in vivo the therapeutic efficacy of doxorubicin against multidrug resistance protein-expressing tumors. Clin Cancer Res 2: 1961–1968. 56 Sharom FJ (1997) The P-glycoprotein efflux pump: how does it transport drugs? J Membr Biol 160: 161–175. 57 Hooijberg JH, Broxterman HJ, Heijn M, Fles DLA, Lankelma J, Pinedo HM (1997) Modulation by (iso)flavonoids of the ATPase activity of the multidrug resistance protein. FEBS Lett 413: 344–348. 58 Taguchi Y, Yoshida A, Takada Y, Komano T, Ueda K (1997) Anti-cancer drugs and glutathione stimulate vanadate-induced trapping of nucleotide in multidrug resistance-associated protein (MRP). FEBS Lett 401: 11–14. 59 Li C, Ramjeesingh M, Wang W, Garami E, Hewryk M, Lee D, Rommens JM, Galley K, Bear CE (1996) ATPase activity of the cystic fibrosis transmembrane conductance regulator. J Biol Chem 271: 28463– 28468. 60 Shapiro A, Ling V (1994) ATPase activity of purified and reconstituted P-glycoprotein from Chinese hamster ovary cells. J Biol Chem 269: 3745– 3754. 940 BioEssays 20.11 61 Chang X, Hou Y-X, Riordan JR (1997) ATPase activity of purified multidrug resistance-associated protein. J Biol Chem 272: 30962–30968. 62 Loe DW, Almquist KC, Cole SPC, Deeley RG (1996) ATPdependent 17-estradiol 17-(-D-glucuronide) transport by multidrug resistance protein: inhibition by cholestatic steroids. J Biol Chem 271: 9683–9689. 63 Jedlitschky G, Leier I, Buchholz U, Hummel-Eisenbeiss J, Burchell B, Keppler D (1997) ATP-dependent transport of bilirubin glucuronides by the multidrug resistance protein MRP1 and its hepatocytes canalicular isoform MRP2. Biochem J 327: 305–310. 64 Evers R, Zaman GJR, van Deemster L, Jansen H, Calafat J, Oomen LCJM, Oude Elferink RPJ, Borst P, Schinkel AH (1996) Basolateral localization and export activity of the human multidrug resistanceassociated protein in polarized pig kidney cells. J Clin Invest 97: 1211–1218. 65 Koike K, Kawabe T, Tanaka T, Toh S, Uchiumi T, Wada M, Akiyama S, Ono M, Kuwano M (1997) A canalicular multispecific organic anion transporter (cMOAT) antisense cDNA enhances drug sensitivity in human hepatic cancer cells. Cancer Res 57: 5475–5479. 66 Flens MJ, Zaman GJR, van der Valk P, Izquierdo MA, Schroeijers AB, Scheffer GL, van der Groep P, de Haas M, Meijer CJLM, Scheper RJ (1996) Tissue distribution of the multidrug resistanceassociated protein. Am J Pathol 148: 1237–1247. 67 Paulusma CC, Bosma PJ, Zaman GJR, Bakker CTM, Otter M, Scheffer GL, Scheper RJ, Borst P, Oude Elferink RPJ (1996) Congenital jaundice in rats with a mutation in a multidrug resistanceassociated protein gene. Science 271: 1126–1128. 68 Roelofsen H, Vos TA, Schippers IJ, Kuipers F, Koning H, Moshage H, Jansen PLM, Muller M (1997) Increased levels of the multidrug resistance protein in lateral membranes of proliferating hepatocytederived cells. Gastroenterology 112: 511–521. 69 Wijnholds J, Evers R, Van Leusden MR, Mol CAAM, Zaman GJR, Mayer U, Beijnen JH, van der Valk M, Krimpenfort P, Borst P (1997) Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance-associated protein. Nat Med 3: 1275–1279. 70 Lorico A, Rappa G, Finch RA, Yang D, Flavell RA, Sartorelli AC (1997) Disruption of the murine MRP (multidrug resistance protein) gene leads to increased sensitivity to etoposide (VP-16) and increased levels of glutathione. Cancer Res 57: 5238–5242. 71 Borst P, Schinkel AH (1996) What have we learnt thus far from mice with disrupted P-glycoprotein genes? Eur J Cancer 32A:985–990. 72 Hipfner DR, Gauldie S, Deeley RG, Cole SPC (1994) Detection of the Mr190,000 multidrug resistance protein, MRP, with monoclonal antibodies. Cancer Res 54: 5788–5792. 73 Hipfner DR, Almquist KC, Stride BD, Deeley RG, Cole SPC (1996) Location of a protease-hypersensitive region in the multidrug resistance protein (MRP) by mapping of the epitope of MRP-specific monoclonal antibody QCRL-1. Cancer Res 56: 3307–3314. 74 Flens MJ, Izquierdo MA, Scheffer GL, Fritz JM, Meijer CJLM, Scheper RJ, Zaman GJR (1994) Immunochemical detection of the multidrug resistance-associated protein MRP in human multidrug-resistant tumor cells by monoclonal antibodies. Cancer Res 54: 4557–4563. 75 Hipfner DR, Gao M, Scheffer G, Scheper R, Deeley RG, Cole SPC (1998) Epitope mapping of monoclonal antibodies specific for the 190 kDa multidrug resistance protein, MRP. Br J Cancer (in press). 76 Hipfner DR, Almquist KC, Leslie EM, Gerlach JH, Grant CE, Deeley RG, Cole SPC (1997) Membrane topology of the multidrug resistance protein, MRP: a study of glycosylation-site mutants reveals an extracytosolic NH2-terminus. J Biol Chem 272: 23623–23630. 77 Kast C, Gros P (1997) Topology mapping of the amino-terminal half of multidrug resistance-associated protein by epitope insertion and immunofluorescence. J Biol Chem 272: 26479–26487. 78 Nelson DA, Bryan J, Wechsler S, Clement JP, Aguilar-Bryan L (1996) The high-affinity sulfonylurea receptor: distribution, glycosylation, purification, and immunoprecipitation of two forms from endocrine and neuroendocrine cell lines. Biochemistry 35: 14793–14799. 79 Gao M, Loe DW, Grant CE, Cole SPC, Deeley RG (1996) Reconstitution of ATP-dependent LTC4 transport by co-expression of both halfmolecules of human MRP in insect cells. J Biol Chem 271: 27782–27787. 80 Gao M, Yamazaki M, Loe DW, Westlake CJ, Grant CE, Cole SPC, Deeley RG (1998) Multidrug resistance protein: identification of regions required for active transport of leukotriene C4. J Biol Chem 273: 10733–10740. 81 Rosenberg MF, Callaghan R, Ford RC, Higgins CF (1997) Structure of the multidrug resistance P-glycoprotein to 2.5 nm resolution determined by electron microscopy and image analysis. J Biol Chem 272: 10685–10694. 82 Rost B, Fariselli P, Casadio R (1996) Topology prediction for helical transmembrane proteins at 86% accuracy. Protein Sci 5: 1704–1718. 83 Loe DW, Deeley RG, Cole SPC (1998) Characterization of vincristine transport by the 190 kDa multidrug resistance protein, MRP: evidence for co-transport with reduced glutathione. Cancer Res 58 (In press Nov 15th issue).