* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Pharmacodynamics
Survey
Document related concepts
Polysubstance dependence wikipedia , lookup
Orphan drug wikipedia , lookup
Pharmaceutical marketing wikipedia , lookup
Compounding wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Psychopharmacology wikipedia , lookup
Drug design wikipedia , lookup
Neuropharmacology wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Pharmacognosy wikipedia , lookup
Drug interaction wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Theralizumab wikipedia , lookup
Prescription costs wikipedia , lookup
Drug discovery wikipedia , lookup
Transcript
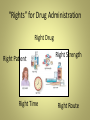
Introduction to the Course of Pharmacology Prof. Ushkalova Elena Andreevna Pharmacology • Basic • Clinical Basic pharmacology An area of medical science which deals with the study of drugs (medicine) that interact with the living system through chemical processes specially by binding to regulatory molecules and activating or inhibiting body processes. Clinical pharmacology is the scientific discipline that involves all aspects of the relationship between drugs and humans. Pharmacology • Parmacokynetics • Pharmacodynamics Movement of the drug in the organism • Where the drug acts • How it acts • The result of its action PHARMACOKINETICS Pharmacodynamics • Pharmacodynamics is the study of the biochemical and physiological effects of drugs and the mechanisms of drug action and the relationship between drug concentration and effect. Pharmacodynamics • • • • Drug actions and effects Sites of action Mechanisms of action Development of therapeutic response: - Onset – the time it takes for the drug to elicit a therapeutic response - Peak - the time it takes for the drug to reach its maximum therapeutic response - Duration - the time the drug concentration is sufficient to elicit a therapeutic response • Agonists - they stimulate and activate the receptors • Antagonists - they stop the agonists from stimulating the receptors Agonists stimulate and activate the receptors Antagonists stop the agonists from stimulating the receptors Causes of Variability in Drug Response • Those related to the conditions of administration 1. Dose, formulation, route of administration. 2. Resulting from repeated administration of drug: drug resistance; drug tolerance-tachyphylaxis; drug allergy (hypersensitivity ) 3. Drug interactions: chemical or physical; GI absorption; protein binding/distribution; metabolism (stimulation/inhibition); excretion (pH/transport processes); receptor (potentiation/antagonism); changes in pH or electrolytes. Factors that Influence Drug Action • Age Pediatric patients and elderly patients may need a reduced dose because of smaller size or inability of liver to metabolize medication • Disease Specific diseases may hinder the pharmacokinetic process of some drugs • Mental State, Genes, Gender Age-related pharmacokinetics Pediatric Age Groups • • • • • • Premature: gestational age < 36 weeks Full-term: gestational age ≥ 36 Neonates: 0–1 months Infants: 1 months – 2 years Children: 2 –12 years Adolescent: 12–16 (18) years Geriatric Considerations • Altered Drug Responses • Adverse Drug Reactions (ADRs) • Polypharmacy • Noncompliance B («best»), C («caution»), D («dangerous») Rational use of drugs The World Health Organization in Nairobi in 1985 defined that, «Rational use of drugs requires that patients receive medications appropriate to their clinical needs, in doses that meet their own individual requirements for an adequate period of time, and the lowest cost to them and their community». • The definition implies that rational use of drugs, especially rational prescribing should meet certain criteria as follows: • appropriate indication • appropriate drug • appropriate patient • appropriate information • appropriate monitoring WHO booklet “Assessing the problems of irrational use of drugs” 2003, ISBN 92 4 156234X 2. Ambwani S & Mathur, A, Rational drug use, health administrator, vol 19, 2006 p.5-7. 3. Sharma HL, Sharma KK. Concept of essential medicine & rational use of drugs in Principle of Pharmacology, Pras publication 2001:9, 106-107 “Rights” for Drug Administration Right Drug Right Patient Right Time Right Strength Right Route Risk/benefit ratio • The benefit-risk balance of a drug must be positive in order to gain and maintain product approval. • Benefits are commonly expressed as the proven therapeutic good of a product, but should also include the patient’s subjective assessment of its effects • Risk is the probability of harm being caused, usually expressed as a percent or ratio of the treated population; the probability of an occurrence • Regulatory authorities accept different degree of risks for different pharmacological classes • It is necessary to monitor benefits and risks during the life cycle of a medicine • The assess of this ratio can be changed: • If new information about this medicine appears • If new drugs with better risk/benefit ratio are approved Efficacy, Effectiveness, Efficiency • Efficacy - the power or capacity to produce a desired result Efficacy is used to express the extent to which a drug works under ideal circumstances (i.e., in clinical trials). • Effectiveness means the capability of producing an effect. Effectiveness is used to express the extent to which a drug works under real world circumstances, i.e., clinical practice (not clinical trials). • Efficiency – economic efficiency (evaluates clinical efficacy/safety/cost of treatment) Side effects occur when drug is used in therapeutic doses, toxic – in supratherapeutic doses ADR Classification • Type A: Augmented pharmacologic effects - dose dependent and predictable • Type B: Bizarre effects (or idiosyncratic) - dose independent and unpredictable • Type C: Develop during long-term therapy (tolerance, addiction, cumulative and withdrawal symptoms) • Type D: Delayed effects (mutagenic, carcinogenic, teratogenic) Therapeutic Window and Therapeutic Ratio • Measure “how safe” drug is • Higher (wider) = safer Therapeutic window is the range of doses of between the amount that gives an effect (effective dose) and the amount that gives more adverse effects than desired effects (minimal therapeutic and minimal toxic doses) Tolerability and Safety • Tolerability - the ability to tolerate drug therapy • Safety – low risk (incidence) of developing serious ADR Serious ADR • • • • • • Life-threatening Requires hospitalization Prolongs hospitalization Causes disability Causes congenital anomalies Causes medically important events Serious Severe International Pharmaceutical Market • More than 8000 INN - International Nonproprietary Names • Annually 150-200 INN are launched Drug Names • Chemical Name – Chemical makeup • Generic name – Name the manufacturer gives a drug (according with «Procedure for the Selection of Recommended International Nonproprietary Names for Pharmaceutical Substances», WHOEB15.R7) – USAN, BAN, DCF, DCIt, JANn etc – Nonproprietary drug, not protected by trademark • Brand name – Trade name – Copyrighted and used exclusively Archie Cochrane (1909–1988) Scottish doctor and epidemiologist , founder of evidence-based medicine His advocacy of randomized controlled trials eventually led to the development of the Cochrane Library database of systematic reviews, the establishment of the UK Cochrane Centre in Oxford and the international Cochrane Collaboration. Basis and History • 1972 г. - Cochrane A L «Effectiveness and Efficiency: Random Reflections on Health Services» • 1993 г. - The Cochrane CollaborationThe Cochrane Library Cochrane Electronic Library • The Cochrane Database of Systematic Reviews (Cochrane Reviews) • The Database of Abstracts of Reviews of Effects (DARE) • The Cochrane Central Register of Controlled Trials (CENTRAL) • The Cochrane Methodology Register (Methodology Register). • Health Technology Assessment Database (HTA). • NHS Economic Evaluation Database (NHS EED) Clinical Trials • Phase I – Studied in 20-100 healthy people (safety) • Phase II – Studied in patients who have the condition it is intended to treat ( efficacy ) • Phase III – Compared to commonly used treatments • Phase IV – Continuation of testing after approved for marketing (effectiveness and safety in real practice) Limitations of Premarketing Clinical Trials • • • • Strict criteria of inclusion/exclusion Homogeneous groups of patients Proper guidance Research is often limited with respect to duration and size. For this reason, in order to demonstrate efficacy, use is often made of surrogate outcome measures and not of clinical endpoints such as a decrease in mortality and morbidity • It is not possible to adequately distinguish rare adverse effects or ones that only occur after long-term use • This ideal-trial situation differs from the real-life situation. In clinical practice medicines are undeniably be used in more heterogeneous groups of patients, often with comorbidity and frequently for longer periods ADR incidence 1:100 1:200 1:1000 1:2000 1:3000 Number of participants 1 ADR 2 ADR (signal) 3 ADR 300 600 3000 6000 30000 480 960 4800 9600 48000 650 1300 6500 13000 65000 Definition of Pharmacovigilance Pharmacovigilance is defined as the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drugrelated problem, including medical errors. The IMPORTANCE of PHARMACOVIGILANCE. Safety Monitoring of medicinal products. World Health Organization 2002 Frances Kelsey (born 1914, July 24) 2010 • FDA Kelsey Award Medical Errors as Important Cause of Morbidity and Mortality • Experts estimate that only in the USA as many as 98,000 people die in any given year from medical errors that occur in hospitals. That's more than die from motor vehicle accidents, breast cancer, or AIDS «(To err is human: building a safer health system» (Washington (DC): National Academy Press, 1999.,An organisation with a memory . London: Department of Health, 2000.) • Several studies have estimated that around 4% to 17% of patients have experienced an adverse event, and that up to half of these incidents could have been prevented Sousa P. Acta Med Port. 2006 Jul-Aug;19(4):309-17. WHY PHARMACOVIGILANCE? • The information collected during the pre-marketing phase of a medical drug is inevitably incomplete with regard to possible adverse reactions : • tests in animals are insufficiently predictive of human safety • in clinical trials patients are selected and limited in number, the conditions of use differ from those in clinical practice and the duration of trials is limited • information about rare but serious adverse reactions, chronic toxicity, use in special groups (such as children, the elderly or pregnant women) or drug interactions is often incomplete or not available. Safety Monitoring of Medicinal Products: Guidelines for Setting Up and Running a Pharmacovigilance Centre. WHO 2000. VigiBase • VigiBase, is the source of worldwide post-marketing case safety reports. • More than 100 countries are currently contributing to the database by submitting ICSRs collected at their national pharmacovigilance centres (NCs) • January 2012 - 7 million ADR reports • April 2012 - 7,160,190 reports • 1997 - Russia was admitted as official member of the WHO Programme VigiBase: Number of Reports (per million inhabitants) from Different Countries (2006-2011) WHO Recommendations • • • • • • • • • Pharmacovigilance is needed in every country, because there are differences between countries (and even regions within countries) in the occurrence of adverse drug reactionsand other drug-related problems. This may be because of differences in: drug production distribution and use (e.g. indications, dose, availability) genetics, diet, traditions of the people pharmaceutical quality and composition (excipients) of locally produced pharmaceutical products the use of non-orthodox drugs (e.g. herbal remedies) which may pose special toxicological problems, when used alone or in combination with other drugs. Data derived from within the country or region may have greater relevance and educational value and may encourage national regulatory decision-making. International monitoring such as the WHO International Drug Monitoring Programme may provide information on possible safety issues which may not yet have emerged within the country‘s data. SAFETY MONITORING of MEDICINAL PRODUCTS. Guidelines for setting up and running a Pharmacovigilance CentreWHO Collaborating Centre for International Drug Monitoring. 2000. ADR Reporting Form