* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download + O2 (g)
History of manufactured fuel gases wikipedia , lookup
Chemical reaction wikipedia , lookup
Biochemistry wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Gas chromatography wikipedia , lookup
Fluorochemical industry wikipedia , lookup
Catalytic reforming wikipedia , lookup
History of chemistry wikipedia , lookup
Aliso Canyon gas leak wikipedia , lookup
History of molecular theory wikipedia , lookup
Abiogenesis wikipedia , lookup
Liquid–liquid extraction wikipedia , lookup
Homoaromaticity wikipedia , lookup
Drug discovery wikipedia , lookup
Stoichiometry wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Microbial metabolism wikipedia , lookup
Physical organic chemistry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
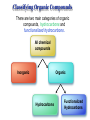
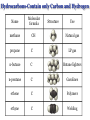
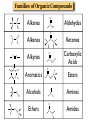
Chapter 3 Chemical Reactions and Equations Chemical Reactions Reactions involve rearrangement and exchange of atoms to produce new pure substances. Reactants Products Chemical Equations Shorthand way of describing a reaction ! Provides information about the reaction ! 1. formulas of reactants and products 2. states of reactants and products 3. relative numbers of reactant and product molecules Combustion of Methane Methane gas reacts with oxygen gas to produce carbon dioxide gas and gaseous water.! CH4(g) + O2(g) ➜ CO2(g) + H2O(g) This equation reads “1 molecule of CH4 gas combines with 1 molecule of O2 gas to make 1 molecule of CO2 gas and 1 molecule of H2O gas.” + + What about conservation of mass ?? + 1C + 4H + + 2O X 1C + 2O +2H + O Combustion of Methane, Balanced To show the reaction obeys the Law of Conservation of Mass, the equation must be balanced. CH4(g) + O2(g) ➜ CO2(g) + H2O(g) ! ! CH4(g) + O2(g) ➜ CO2(g) + 2 H2O(g) ! ! CH4(g) + 2 O2(g) ➜ CO2(g) + 2 H2O(g) + + “1 molecule of CH4 gas combines with 2 molecules of O2 gas to make 1 molecule of CO2 gas and 2 molecules of H2O gas.” Symbols Used in Equations Symbols used to indicate state after chemical: ! (g) = gas; (l) = liquid; (s) = solid (aq) = aqueous = dissolved in water ! Energy symbols used above the arrow for conditions for reactions: ! Δ = heat hν = light shock = mechanical elec = electrical Steps in Balancing Equations 1. In compounds balance elements other than H and O. ! a. Balance elements which occur only once on each side of the equation. b. Start with the elements which occur the most. c. Balance polyatomic ions which do not change in the reaction. 2. Be prepared to rebalance if something changes!!! ! 3. Balance H. ! 4. Balance O. ! 5. Balance elements which appear in their “elemental” forms. When aluminum metal reacts with air, it produces a white, powdery compound, aluminum oxide.! aluminum(s) + oxygen(g) ➜ aluminum oxide(s) ! Al(s) + O2(g) ➜ Al2O3(s) ! 2 Al(s) + O2(g) ➜ Al2O3(s) ! 2 Al(s) + 3 O2(g) ➜ 2 Al2O3(s) ! 4 Al(s) + 3 O2(g) ➜ 2 Al2O3(s) Solid phosphorous (P4) reacts with hydrogen gas to produce phosphorous trihydride. P4 (s) ! P4 (s) ! P4 (s) + H2 (g) ----------> PH3 (g) + H2 (g) ----------> 4 PH3 (g) + 6 H2 (g) ----------> 4 PH3 (g) Solid potassium chlorate decomposes to produce ! oxygen gas and potassium chloride. KClO3 (s) -------------------> 2 KClO3 (s) -------------------> ! 2 KClO3 (s) -------------------> O2 (g) + KCl (s) 3 O2 (g) + KCl (s) 3 O2 (g) + 2 KCl (s) Aqueous sulfuric acid reacts with solid sodium cyanide! to produce aqueous sodium sulfate and hydrogen cyanide gas. H2SO4 (aq) + NaCN (s) ------> Na2SO4 (aq) + HCN(g) ! H2SO4 (aq) + 2 NaCN (s) ------> Na2SO4 (aq) + HCN(g) ! H2SO4 (aq) + 2 NaCN (s) ------> Na2SO4 (aq) + 2 HCN(g) Aqueous potassium phosphate reacts with aqueous calcium nitrate! to produce solid calcium phosphate and aqueous potassium nitrate. ! ! ! K3PO4 (aq) + Ca(NO3)2 (aq) ----------> Ca3(PO4)2 (s) + KNO3 (aq) K3PO4 (aq) + 3 Ca(NO3)2 (aq) ----------> Ca3(PO4)2 (s) + KNO3 (aq) K3PO4 (aq) + 3 Ca(NO3)2 (aq) ----------> Ca3(PO4)2 (s) + 6 KNO3 (aq) 2 K3PO4 (aq) + 3 Ca(NO3)2 (aq) ----------> Ca3(PO4)2 (s) + 6 KNO3 (aq) Write a balanced equation for the combustion of butane, C4H10. C4H10 (g) + O2 (g) ➝ CO2 (g) + H2O (g) ☚ C4H10 (g) + O2 (g) ➝ 4 CO2 (g) + H2O (g) C4H10 (g) + O2 (g) ➝ 4 CO2 (g) + 5 H2O (g) ! ! ! C4H10 (g) + 13/2 O2 (g) ➝ 4 CO2 (g) + 5 H2O (g) ! 2 C4H10 (g) + 13 O2 (g) ➝ 8 CO2 (g) + 10 H2O (g) Acetic acid reacts with the metal aluminum to make aqueous aluminum acetate and gaseous hydrogen Al(s) + HC2H3O2(aq) ➜ Al(C2H3O2)3(aq) + H2(g) Al(s) + 3 HC2H3O2(aq) ➜ Al(C2H3O2)3(aq) + H2(g) Al(s) + 6 HC2H3O2(aq) ➜ Al(C2H3O2)3(aq) + 3 H2(g) Al(s) + 6 HC2H3O2(aq) ➜ 2 Al(C2H3O2)3(aq) + 3 H2(g) 2 Al(s) + 6 HC2H3O2(aq) ➜ 2 Al(C2H3O2)3(aq) + 3 H2(g) Organic Chemistry Classifying Compounds Organic vs. Inorganic In the18th century, compounds from living things were called organic; compounds from the nonliving environment were called inorganic. ! Organic compounds easily decomposed and could not be made in the 18th-century lab. ! Inorganic compounds were very difficult to decompose, but could be synthesized. Modern Classification of Compounds Organic vs. Inorganic Today we commonly make organic compounds in the lab and find them all around us. ! Organic compounds are mainly made of C and H, sometimes with O, N, P, S, halogens, and trace amounts of other elements. ! The main element that is the focus of organic chemistry is carbon. Carbon Bonding in Organic Compounds Carbon atoms bond almost exclusively covalently in organic compounds. ! When C bonds, it forms four covalent bonds. Carbon is unique in that it can form limitless chains of C atoms, both straight and branched, and rings of C atoms. Classifying Organic Compounds There are two main categories of organic compounds, hydrocarbons and functionalized hydrocarbons. All chemical compounds Inorganic Organic Hydrocarbons Functionalized Hydrocarbons Hydrocarbons-Contain only Carbon and Hydrogen Name Molecular formula methane CH propane C LP gas n-butane C Butane lighters n-pentane C Gasolines ethene C Polymers ethyne C Welding Structure Use Natural gas Families of Organic Compounds C O Alkanes C C Aldehydes H O C C C C C C Alkenes C C Aromatics C C O H C O C C O O C C O Alcohols Ethers Ketones C O Alkynes C C C Esters Amines N O C H Carboxylic Acids N Amides