* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Classification of colorectal cancer based on correlation of clinical
Artificial gene synthesis wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
History of genetic engineering wikipedia , lookup
Epigenetic clock wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
DNA methylation wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Epigenomics wikipedia , lookup
Frameshift mutation wikipedia , lookup
Bisulfite sequencing wikipedia , lookup
Microevolution wikipedia , lookup
Genome (book) wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
BRCA mutation wikipedia , lookup
Epigenetics in stem-cell differentiation wikipedia , lookup
Microsatellite wikipedia , lookup
Point mutation wikipedia , lookup
Mir-92 microRNA precursor family wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Histopathology 2007, 50, 113–130. DOI: 10.1111/j.1365-2559.2006.02549.x REVIEW Classification of colorectal cancer based on correlation of clinical, morphological and molecular features J R Jass Department of Pathology, McGill University, Montreal, Canada Jass J R (2007) Histopathology, 50, 113–130 Classification of colorectal cancer based on correlation of clinical, morphological and molecular features Over the last 20 years it has become clear that colorectal cancer (CRC) evolves through multiple pathways. These pathways may be defined on the basis of two molecular features: (i) DNA microsatellite instability (MSI) status stratified as MSI-high (MSI-H), MSI-low (MSI-L) and MS stable (MSS), and (ii) CpG island methylator phenotype (CIMP) stratified as CIMPhigh, CIMP-low and CIMP-negative (CIMP-neg). In this review the morphological correlates of five molecular subtypes are outlined: Type 1 (CIMP-high ⁄ MSI-H ⁄ BRAF mutation), Type 2 (CIMP-high ⁄ MSI-L or MSS ⁄ BRAF mutation), Type 3 (CIMP-low ⁄ MSS or MSI-L ⁄ KRAS mutation), Type 4 (CIMP-neg ⁄ MSS) and Type 5 or Lynch syndrome (CIMP-neg ⁄ MSI-H). The molecular pathways are determined at an early evolutionary stage and are fully established within precancerous lesions. Serrated polyps are the precursors of Types 1 and 2 CRC, whereas Types 4 and 5 evolve through the adenoma–carcinoma sequence. Type 3 CRC may arise within either type of polyp. Types 1 and 4 are conceived as having few, if any, molecular overlaps with each other, whereas Types 2, 3 and 5 combine the molecular features of Types 1 and 4 in different ways. This approach to the classification of CRC should accelerate understanding of causation and will impact on clinical management in the areas of both prevention and treatment. Keywords: cancer, classification, colorectal, DNA methylation, microsatellite instability, pathways Abbreviations: CIMP-high, -low or -neg, CpG island methylator phenotype-high, -low, or negative; CIN, chromosomal instabilty; CRC, colorectal cancer; MSI, microsatellite instability; MSI-H, microsatellite instability-high; MSI-L, microsatellite instability-low; MSS, microsatellite stable Introduction The role of the histopathologist is no longer limited to issuing an accurate tissue diagnosis but is increasingly directed towards the provision of prognostic information and additional findings directly relevant to patient management. This ongoing refinement of reporting practice should not obscure the more fundamental role of the pathologist in the classification of disease. Address for correspondence: Jeremy R Jass, Department of Pathology, McGill University, Duff Medical Building, 3775 University Street, Montreal, Quebec H3A 2B4, Canada. e-mail: [email protected] 2007 The Author. Journal compilation 2007 Blackwell Publishing Limited. Classification is more than the mere naming of disease entities or even the collation of their particular diagnostic features. It includes the elucidation of clinicopathological correlation, which is the starting point for the investigation of the causation, evolution and natural history of a disease. It is necessary for a disease to be properly classified in order to achieve effective clinical management and meaningful laboratory investigation of the underlying mechanisms. The classification of cancer has traditionally been based mainly on microscopic morphology supplemented, in more complex forms of malignancy, by immunophenotyping and, more rarely, molecular approaches. Molecular technology has been mainly limited to subtle 114 J R Jass refinements of classification, particularly when markers are shown to contribute prognostic information or predict chemoresponsiveness. In the case of colorectal cancer (CRC), both clinical management and research have proceeded for many decades on the basis that CRC is a homogeneous entity. Nevertheless, particular morphological subtypes, such as mucinous carcinoma, have long been recognized and clinical features have been shown to differ according to anatomical subsite.1 The evolution of CRC was also understood to proceed on the basis of a relatively uniform and linear sequence of steps, with APC inactivation initiating adenomas and additional genetic changes, notably KRAS mutation, and TP53 inactivation promoting the emergence of increasingly aggressive subclones.2 The condition familial adenomatous polyposis (FAP), caused by germ-line mutation of APC, was perceived as the hereditary counterpart of the ‘vast majority’ of sporadic CRCs.3 While the mutational events driving tumorigenesis were deemed to be selected on the basis that each would confer a biological advantage,4 an additional factor was required to explain how the accumulation of multiple genetic changes could occur within the limited lifespan of a cell. This additional factor, known as genetic instability, implicates the loss of a mechanism (or mechanisms) not only critical for the maintenance of genomic fidelity during cell division but also capable of triggering apoptosis in the setting of accumulating genetic damage.5 Types of genetic instability The condition FAP illustrates the requirement for genetic instability. Without this ingredient many thousands of adenomas may be initiated by inactivation of APC, but the fate of the vast majority is merely to grow harmlessly over several decades. In the context of sporadic CRC an individual adenoma would appear (on the basis of the relative frequency of adenoma versus carcinoma) to have a much higher risk of malignant transformation.6 The concept that lesions of similar appearance could have markedly different biological properties was highlighted by a second form of familial CRC: Lynch syndrome, known also as hereditary nonpolyposis colorectal cancer. In this condition it is evident that a high proportion of adenomas will, if left untreated, progress to CRC and do so within a short timeframe.7 Most adenomas in subjects with Lynch syndrome show loss of expression of a DNA mismatch repair protein (usually MLH1 or MSH2) and display a form of genetic instability characterized by the accumulation of numerous mutations which specifically target repetitive sequences of DNA.8 These sequences occur most frequently in non-encoding microsatellite regions, hence the term microsatellite instability (MSI). Following inactivation of a DNA mismatch repair gene one may detect such mutations at a high frequency throughout the genome, hence the term MSI-high (MSI-H). MSI-low (MSI-L) will be discussed below. Because short repetitive sequences also occur within the encoding regions of certain tumour suppressor genes such as TGFbRII, IGF2R and BAX, these may be mutated and inactivated.9–11 CRCs with MSI have a diploid DNA content with few losses or gains of chromosomal regions.12 Genetic instability was therefore conceived as operating on two levels, a more subtle level affecting DNA sequences (MSI-H), and chromosomal instability (CIN) affecting whole chromosomes or parts of chromosomes.13 These forms of instabilty are mutually exclusive, so that CRCs with CIN will be MS stable (MSS). Notwithstanding the mutual exclusivity of these two forms of genetic instability, all CRCs were considered to evolve through a similar linear sequence of genetic alterations. Indeed, APC, KRAS and TP53 were all shown to be mutated in CRCs with MSI-H from patients with Lynch syndrome and in malignant cell lines with MSI-H.14–20 Need for an alternative pathway to explain sporadic CRCs with MSI-H Support for the existence of two largely independent pathways to sporadic CRC was slow to develop. Acceptance of such a notion had to supplant an attractive and elegant paradigm, in which APC inactivation and loss of DNA mismatch repair were envisaged to initiate and promote (respectively) an essentially similar evolutionary pathway in both sporadic and familial settings.13 There was no obvious imperative for an alternative pathway to sporadic MSI-H CRC on the basis of the literature amassed within basic science journals. Notably, sporadic colorectal adenomas could show MSI-H,21 whereas a similar spectrum of somatic mutations occurred in CRC cell lines regardless of microsatellite status.20 Why complicate the picture by introducing hyperplastic polyps (or closely related lesions) when these lesions had been dismissed as harmless for decades?22 Why suggest that sporadic and Lynch syndrome-associated MSI-H CRCs are not in fact direct counterparts?23 absence o f e xpected g enetic signatures The reluctance to counter the status quo meant that reports providing contrary data were either ignored or 2007 The Author. Journal compilation 2007 Blackwell Publishing Ltd, Histopathology, 50, 113–130. Clasification of colorectal cancer rebutted with tendentious arguments. For example, with the single exception noted above,21 MSI-H consistent with DNA mismatch repair deficiency was rarely observed in sporadic adenomas24 and the few such examples found turned out to be mainly derived from patients with Lynch syndrome.25 These findings gave rise to the suggestion that, unlike the adenomas in Lynch syndrome, MSI-H must occur as a relatively late event in sporadic adenomas.21 Why, it may then be asked, were the genetic alterations associated with initiation and early progression of sporadic adenomas not found in sporadic MSI-H CRC? For example, in studies that carefully distinguished sporadic MSI-H CRC and Lynch syndrome, the sporadic MSI-H subset showed infrequent APC mutation or loss of the APC locus on chromosome 5q, while KRAS mutation was also rare.26–28 These observations were countered by the argument that alterations in other components of the Wnt signalling pathway could substitute for APC inactivation, notably an activating mutation of CTNNB1 (encodes b-catenin). While it is certainly correct that CTNNB1 is sometimes mutated in CRC with MSI-H,29 this mutation is mainly limited to Lynch syndrome cancers,30,31 whereas it is absent or very rarely detected in sporadic MSI-H CRC.26,31,32 The non-involvement of APC and CTNNB1 in sporadic MSI-H CRC is fully supported by the immunoexpression pattern for b-catenin, in which the normal distribution along lateral cell membranes is maintained while aberrant translocation to the nucleus is infrequent.27,33 A single study from Japan linking mutation of CTNNB1 with sporadic MSI-H CRC has not been confirmed in Western populations.34 In fact, a subsequent study from the same group in Japan showed that mutation of CTNNB1 was negatively associated with both BRAF mutation and methylation of MLH1, which are the hallmark genetic alterations in sporadic MSI-H CRC.35 The finding of CTNNB1 mutation in earlyonset cases of MSI-H CRC29 could be due to either Lynch syndrome or germ-line hemi-allelic methylation of MLH1.36 The over-representation of CTNNB1 mutations in MSI-H CRC cell lines37 is probably due to the fact that very few such cell lines are derived from sporadic MSI-H CRCs. Methylation of the APC promoter could fill the mutational gap in theory, but this epigenetic change occurs in only 18% of CRCs, may affect the wild-type allele when there is already an APC mutation, and is not associated with either MSI-H or with methylation of other genes.38 Furthermore, it has been shown that at least one APC allele must be retained in a truncated form to drive proliferation and tumorigenesis.39 This indicates that bi-allelic methylation of APC (leading to complete silencing) may not provide an important 115 growth advantage. Invoking other components of the Wnt signalling pathway such as AXIN240 or TCF441 in the initiation of sporadic MSI-H neoplasia does not provide a surrogate directly equivalent to APC inactivation, since these genes are mutated at a relatively late stage (after the acquisition of MSI-H status). The widely accepted notion that other components of the ‘canonical’ Wnt pathway can be invoked in the initiation of the subset of CRCs without APC mutation is unproven. presence of unexp ect ed genetic s ignatures In addition to the absence of adenoma-specific mutations, sporadic MSI-H CRCs are characterized by alterations, specifically extensive DNA methylation and BRAF mutation, that are not only rare in sporadic adenomas42–45 but are also not observed in Lynch syndrome CRC.46,47 The association between BRAF mutation and CIMP has been shown to be extremely strong in CRC with an odds ratio of over 200.48 While DNA methylation may occur in sporadic adenomas,49 it is seldom marked in small tubular adenomas,50 although it may implicate more loci in adenomas with high-grade dysplasia and ⁄ or villous change.51 By contrast, very extensive DNA methylation is the usual finding in serrated polyps occurring in the proximal colon52,53 that also show frequent BRAF mutation.44 The most convincing evidence for the existence of a serrated pathway to MSI-H CRC is the direct observation of a serrated polyp–dysplasia–carcinoma transition supported by immunohistochemical and molecular correlation. This has been achieved by the demonstration of MLH1 loss in dysplastic or malignant subclones and the presence of MSI-H in the DNA extracted from such subclones.54–57 Methylation of the MLH1 promoter is the principal mechanism underlying the silencing of MLH1 and loss of mismatch repair proficiency in sporadic MSI-H CRC.58 However, based on the spectrum of genetic alterations in serrated polyps, these lesions must also serve as the principal source of sporadic MSI-H CRC, whereas the conventional adenoma–carcinoma sequence initiated by APC or CTNNB1 mutation and subsequently driven by KRAS mutation is more likely to be associated with the early evolution of CRC in Lynch syndrome. Heterogeneity of sporadic MSS CRC: stratification based on DNA methylation and low-level MSI Removal of the two forms of MSI-H CRC (familial and sporadic) leaves the large MSS subset comprising 2007 The Author. Journal compilation 2007 Blackwell Publishing Ltd, Histopathology, 50, 113–130. 116 J R Jass around 85% of CRC. It might be supposed that this group is homogeneous at the molecular level and comprises CRC with mutation of APC, KRAS and TP53. In practice, however, only around 10% of CRCs are characterized by this ‘classic’ genotype.59,60 Not only is the MSS group highly heterogeneous, but it includes some CRCs with molecular features that characterize the sporadic MSI-H subset, notably BRAF mutation,44,61 extensive DNA methylation or the CpG island methylator phenotype (CIMP),62–66 and diploid status or chromosomal stability.67–70 It is notable that MSS CRCs with high-level CIMP and ⁄ or BRAF mutation also share certain clinical and pathological features with the sporadic MSI-H subset with CIMP. These features include: (i) a predilection for females,61,63,66 (ii) increased age at onset,66 (iii) a predilection for proximal colon,61–63,65,66 (iv) poor differentiation,61,63,65,66 (v) mucinous differentiation61–63,65,66,71 and (vi) round and vesicular nuclei with a prominent nucleolus.62 However, there are also differences from the sporadic MSI-H subset with CIMP, including: (i) a higher incidence of presentation at an advanced pathological stage,61,63,65,66 (ii) infiltrative growth pattern with discohesive tumour cells,65 (iii) lack of tumour-infiltrating lymphocytes (TILs),62,63 (iv) poor prognosis64,72 and (v) responsiveness to adjuvant treatment with 5-fluorouracil.64 MSS CRCs with BRAF mutation and ⁄ or DNA methylation are likely to show a degree of overlap with MSS CRC with diploid DNA status or infrequent loss of heterozygosity. For example, MSS CRCs with diploid DNA content and ⁄ or little evidence of CIN have been shown to be more frequent in the proximal colon,68,69 to present at an advanced stage68 and to be mucinous and ⁄ or poorly differentiated.70 Furthermore, concordant silencing of multiple tumour suppressor genes through promoter region methylation would explain how neoplasia may develop without a background of either MSI or CIN. s i g n i f i c a n c e o f l o w - l e v e l ms i While the MSS group lacks MSI-H by definition, a subset of non-MSI-H CRC shows MSI-L. The concept of MSI-L has been controversial and CRCs with MSI-L do not represent a clearly defined group. Nevertheless, there is now good evidence that MSI-L status occurs as a non-random and biologically based phenomenon and is not merely a polymerase chain reaction-based artefact.73,74 MSI-L CRCs were distinguished from both MSI-H and MSS CRCs on the basis of gene expression profiles75 and also differ from MSS CRCs in showing frequent instability in the trinucleotide repeat region of RAS-induced senescence 1 (RIS1).76 MSI-L status has been shown to be an independent adverse prognostic feature in stage III CRC from patients not treated with adjuvant chemotherapy77,78 and particularly when occurring in association with mutation of RIS1.76 MSI-L CRCs were found to be over-represented among CIMP-high CRCs that were not MSI-H.79 Additionally, CRCs with both KRAS mutation and MSI-L showed more extensive DNA methylation than MSS CRCs with KRAS mutation or non-MSI-H CRCs without either KRAS or BRAF mutation.80 While the preceding points might link MSI-L with both DNA methylation and diploid DNA status, one study has shown that MSI-L CRC in fact had higher rates of loss of heterozygosity than the MSS group.69 Two mechanisms for MSI-L status have been advanced: (i) increased generation of methylG:T mismatches due to loss of expression of 0-6-Methylguanine DNA Methyltransferase (MGMT) that would stress the DNA mismatch repair machinery,81 and (ii) partial methylation and loss of expression of the DNA mismatch repair gene MLH1.82,83 These mechanisms might also synergise and account for the high end of the range of MSI-L or ‘super-low’ status.73 Involvement of MLH1 (partial methylation) alone might result in MSI-L without chromosomal instability or KRAS mutation. Involvement of MGMT (with or without MLH1) would be associated with KRAS mutation84 and chromosomal instability on the basis that methylG:T mismatches give rise to futile cycles of DNA excision and attempted repair that may culminate in chromosomal damage.85,86 Methylation of MGMT was found to be most frequent in the subset of CRC with both MSI-L status and KRAS mutation.80 h e t e r o g e n e i t y wi t h i n ci m p Differences between CIMP-high and CIMP-low may not be merely quantitative. CIMP-high CRCs have frequent BRAF mutation and show methylation of many markers, consistent with a generalized increase in de novo methylation (described as CIMP1).87,88 By contrast, CIMP-low CRCs have very frequent KRAS mutation (92%) and show a denser pattern of methylation affecting a smaller number of genes, suggesting an epigenetic defect influencing the spread of methylation from methylation centres (described as CIMP2).87,88 It is likely that synergy between BRAF or KRAS mutations and particular patterns of DNA methylation is necessary to bring about early tumorigenic events. Activated ras and raf have been linked to cell senescence characterized by irreversible cell cycle arrest.89,90 Interestingly, hyperplastic and closely related polyps initiated by either KRAS or BRAF mutation 2007 The Author. Journal compilation 2007 Blackwell Publishing Ltd, Histopathology, 50, 113–130. Clasification of colorectal cancer (see below) have been traditionally linked with cell senescence.91,92 A tumorigenic effect requires the additional inactivation of tumour suppressor genes normally associated with cell cycle arrest, such as CDKN2A (encodes p16), p14ARF and TP53.89,90 This could explain the association between KRAS mutation and methylation of CDKN2A and ⁄ or p14ARF in subsets of CRC.80 In the case of BRAF, it has been suggested that more widespread methylation of pro-apoptotic genes such as RASSF1, RASSF2, NORE1 (RASSF5) and MST1 is required to bring about a tumorigenic effect.93 Genes that happen to be methylated in colon and other cell lines not only share distinct functional properties (cell signalling, cell adhesion, cell–cell communication and ion transport) but have common sequence motifs in their promoters.94 This suggests that de novo methylation is not a random process but occurs through a specific instructive mechanism.94 The evidence for a genetic basis for CIMP is outlined in the following sections. me ch an is m s f or ci mp As well as the strong association with BRAF mutation, subjects with CIMP-high or CIMP1 CRC are more likely to have a positive family history of CRC. In a large population-based study in which subjects were selected on the basis of having MSS CRC with BRAF mutation, the odds ratio for a positive family history compared with patients with MSS ⁄ BRAF-negative CRC was 4.23 (95% confidence interval 1.65, 10.84).61 Among subjects with MSI-H CRC, BRAF mutation was a negative predictor for a positive family history.61 However, subjects with MSI-H ⁄ BRAF-negative CRC were relatively young and many would be expected to be from Lynch syndrome families. When the same population-based set of cases was studied with respect to CIMP and family history, the link was less strong.66 However, this analysis employed a cutoff for CIMP in which only about one-third of ‘CIMPpositive’ CRCs had BRAF mutation. The hereditary link appears to be with CIMP-high and ⁄ or BRAF mutation. Two high-risk family clinic-based studies have suggested that patients with CIMP CRC or BRAFpositive CRC may represent a new cancer family syndrome with an increased risk of extracolonic as well as colorectal malignancy.95,96 A third clinic-based study identified Lynch syndrome-like families in which CRCs showed variable MSI status with combinations of MSS, MSI-L and MSI-H CRC.97 In Lynch syndrome all tested CRCs would be expected to be MSI-H, whereas in the MSI-variable families most of the CRCs were either MSS or MSI-L. In these families, about half of 117 which met the Amsterdam criteria, a high proportion of both polyps and CRC showed mutation of BRAF and ⁄ or methylation of the CIMP marker MINT31. Many of the polyps were advanced serrated polyps (serrated adenomas or mixed polyps) and two family members had hyperplastic polyposis.97 One hospitalbased study found no increased family history of cancer in subjects with CIMP CRC. However, this study excluded families meeting unspecified criteria for Lynch syndrome and used a loose definition of CIMP.98 The preceding studies suggest that there is likely to be a genetic predisposition to DNA methylation which results in polyps and CRC with CIMP-high (CIMP1). This is supported by the finding of extensive DNA methylation in the normal colorectal mucosa in three unrelated subjects with hyperplastic polyposis.93 Some patients with hyperplastic polyposis develop multiple CRCs that may be MSS, MSI-L and MSI-H within the same subject.55 Conceivably, hyperplastic polyposis is inherited as an autosomal recessive disorder associated with multiple polyps and cancers. Subjects with a single copy of the altered gene may develop small numbers of serrated polyps and be at increased risk of developing CIMP CRC. The early evolution of CIMP CRC may be the same regardless of MSI status. Modifying genetic factors may then affect the likelihood of methylation and inactivation of MGMT or MLH1, which will in turn determine whether the pathway diverges to give CRCs that are MSS, MSI-L or MSI-H.93 CIMP-high or BRAF-positive CRCs may share an underlying genetic predisposition and constitutional factors, as indicated by the association with female gender. In addition, particular environmental factors may be important in the pathogenesis of these CRCs. The increased risk of CRC associated with smoking is largely explained by the subset with BRAF mutation and ⁄ or CIMP.99 Smoking is also associated with hyperplastic polyps, suggesting that the increased risk is related to the earliest evolutionary steps.100 Interestingly, a polymorphism in the promoter region of MLH1 (93GfiA) modifies the risk of hyperplastic polyps (mainly left-sided) in smokers and raises the possibility of a gene–environment interaction that could predispose to partial methylation of MLH1 and an MSI-L ⁄ CIMP-low pathway (see above).101 Chronic inflammation in the context of ulcerative colitis has also been linked with DNA methylation.102 link between l oss of imprint ing a nd cimp Genomic imprinting occurs through methylation of one allele so that a gene is expressed only through the 2007 The Author. Journal compilation 2007 Blackwell Publishing Ltd, Histopathology, 50, 113–130. J R Jass CIM P- H MSIH 5 1 2 3 -L 4 S M S/ MS CI non-imprinted (usually paternal) allele. IGF2 is one of the more well-known imprinted genes. Loss of imprinting (LOI) of IGF2 has been asociated with MSI-H CRC.103 A study from Japan failed to show this link but found that CRCs with LOI had the morphological features of CRCs with CIMP, notably poor differentiation, mucinous differentiation and proximal location.104 The link between LOI and CIMP may be explained by the fact that LOI depends upon the methylation of a controlling element known as the H19 differential methylated region.105 The fact that LOI of IGF2 may be found in normal colonic epithelium and even normal leucocytes as well as CRC106 suggests that the H19 differential methylated region is exceptionally sensitive to methylation pressures. The observation that IGF2 LOI in normal leucocytes is associated with a personal and family history of CRC106 provides additional evidence for an inherited basis for CIMP. CI MP 118 M I-L PNe g Molecular classification of colorectal cancer It would undoubtedly be more convenient for cancer researchers if CRC could be viewed as a homogeneous disorder, because an individual CRC or cell line could then be considered representive of all CRC. At one level this may still be true, insofar as the acquisition of the full malignant phenotype probably depends upon the combined disruption of all the major signalling pathways. Indeed, while it has been argued above that familial and sporadic MSI-H CRC evolves through different pathways, there is very considerable overlap in the altered gene expression signatures of these two types of CRC, as shown by microchip array-based analysis.107 However, this does not refute the concept that the pathways differ at a fundamental level. Rather, it highlights major limitations of present-day biotechnology insofar as it is incapable of either explaining the evolutionary history of a malignancy or resolving subtle differences in levels of gene expression existing at the key control points of signalling pathways. Based primarily on: (i) the underlying types of genetic instability, and (ii) the presence of DNA methylation, the following five molecular subtypes of CRC (with approximate frequencies) are suggested: 1 CIMP-high, methylation of MLH1, BRAF mutation, chromosomally stable, MSI-H, origin in serrated polyps, known generally as sporadic MSI-H (12%). 2 CIMP-high, partial methylation of MLH1, BRAF mutation, chromosomally stable, MSS or MSI-L, origin in serrated polyps (8%). 3 CIMP-low, KRAS mutation, MGMT methylation, chromosomal instability, MSS or MSI-L, origin in adenomas or serrated polyps (20%). Figure 1. Derivation of molecular colorectal cancer groups 1–5 based on CpG island methylator phenotype (CIMP) status (H, high; L, low; Neg, negative) and DNA microsatellite instability (MSI) status (H, high; L, low; S, stable). 4 CIMP-negative, chromosomal instability, mainly MSS, origin in adenomas (may be sporadic, FAPassociated or MUTYH (formerly MYH) polyposis associated108) (57%). 5 Lynch syndrome, CIMP-negative, BRAF mutation negative, chromosomally stable, MSI-H, origin in adenomas (3%) (described also as familial MSI-H CRC in this review). Sporadic MSI-H CRCs are deliberately termed as group 1 because they are the most obviously homogeneous group with respect to their clinical, morphological and molecular features. However, group 5 CRCs share features with group 1 CRCs and these groups may be conceived as completing a circle rather than representing the ends of a spectrum (Figure 1). Overlaps between the groups are not excluded. For example, KRAS rather than BRAF mutation may occasionally occur in association with CIMP-high.48 Morphological correlations While particular morphological correlates have been demonstrated for each of the preceding subtypes, it is not necessarily possible to recognize each group on the basis of morphological features alone. In particular, there are no studies of the morphological distinction of groups 3 and 4. It is often the case that a particular feature will characterize two or more groups. The 2007 The Author. Journal compilation 2007 Blackwell Publishing Ltd, Histopathology, 50, 113–130. Clasification of colorectal cancer Table 1. Molecular, clinical and morphological features of colorectal cancer groups 1–5 119 Feature Group 1 Group 2 Group 3 Group 4 Group 5 MSI status H S⁄L S⁄L S H Methylation +++ +++ ++ +⁄– +⁄– Ploidy Dip > An Dip > An An > Dip An > Dip Dip > An APC +⁄– +⁄– + +++ ++ KRAS – + +++ ++ ++ BRAF +++ ++ – – – TP53 – + ++ +++ + Location R>L R>L L>R L>R R>L Gender F>M F>M M>F M>F M>F Precursor SP SP SP ⁄ AD AD AD Serration +++ +++ + +⁄– +⁄– Mucinous +++ +++ + + ++ Dirty necrosis + + ? +++ + Poor differentiation +++ +++ + + ++ Circumscribed +++ + ? ++ ++ Tumour budding +⁄– + ? +++ + Lymphocytes +++ + ? + +++ MSI, microsatellite instability; H, high; S, stable; L, low; Dip, diploid; An, aneuploid; Serration, serrated morphology; SP, serrated polyp; AD, adenoma; Circumscribed, circumscribed invasive margin. primary basis for the classification of CRC is therefore molecular. In this section the focus will be on the various discriminating morphological features and the extent to which they cut across the molecular subtypes. An overview of the morphological findings in CRC groups 1–5 is shown in Table 1. se r ra t e d m o rp h o l og y The term ‘serrated adenocarcinoma’ was introduced to describe CRC with such a close structural and functional (histochemical) resemblance to the hyperplastic polyp that it was difficult to dismiss a direct histogenetic relationship between the two (Figure 2a).109 Serrated adenocarcinomas were subsequently described in association with multiple serrated adenomas and hyperplastic polyps110 and finally in considerable detail when observed either with or without a contiguous serrated adenoma (Figure 2b).56,82 It should be strongly stressed that glandular serration in isolation is a non-specific feature that may be produced by branching and folding of proliferating epithelium that occurs in CRC regardless of early histogenesis. Serrated adenocarcinomas are recognized by the presence of additional features which include: (i) cribriform, lacelike and trabecular structures, (ii) secretion of intracellular and often abundant extracellular mucin, (iii) a low nuclear:cytoplasmic ratio, (iv) round or ovoid nuclei that are vesicular with a prominent nuclear membrane (chromatin condensation at the nuclear membrane) and large nucleolus, (v) well-preserved nuclear polarity, and (vi) an overall ‘pink’ appearance due to relatively abundant eosinophilic cytoplasm and lack of nuclear hyperchromatism.82 Serrated morphology has been linked with MSI, but the association was significant for MSI-L CRC, with only a trend for MSI-H CRC.82 Many of the structural and cytological features accompanying serrated morphology are linked with DNA methylation. However, glandular serration in isolation was not shown to be associated with CIMP,65 emphasizing the importance of a more global appraisal. Nevertheless, glandular serration was more frequent 2007 The Author. Journal compilation 2007 Blackwell Publishing Ltd, Histopathology, 50, 113–130. 120 J R Jass a b c d e f Figure 2. Colorectal cancers with serrated morphology and serrated precursor lesions. Serrated adenoma (a) that was contiguous with a microsatellite instability-high serrated adenocarcinoma (group 1) (b). Serration is not as obvious in the carcinoma (b) as the serrated adenoma (a) but the carcinoma shows other features of serrated morphology, including abundant eosinophilic cytoplasm, ovoid nuclei and extracellular mucin. Sessile serrated adenoma showing branched, dilated and back-to-back glands but no cytological atypia (c). Poorly differentiated group 2 carcinoma [d, H&E; e, immunohistochemistry for Methylguanine DNA Methyltransferase (MGMT); f, immunohistochemistry for MLH1]. The glands to the left are normal and show nuclear expression of both MGMT and MLH1. The gland with features of serrated adenoma and the poorly differentiated adenocarcinoma infiltrating the lamina propria show loss of nuclear expression of MGMT but not MLH1 (ABC technique). 2007 The Author. Journal compilation 2007 Blackwell Publishing Ltd, Histopathology, 50, 113–130. Clasification of colorectal cancer 121 in CRC from members of MSI-variable families in which the CRCs showed frequent BRAF mutation and ⁄ or methylation of the CIMP marker MINT31.97 A serrated morphology will therefore be over-represented among group 1 and 2 CRCs and may help to distinguish sporadic from familial (Lynch syndrome) MSI-H CRC.111 compared with serrated polyps.116 KRAS mutation is closely linked to villous change and dysplasia (but not size) and is found in some mixed polyps and serrated adenomas as well as conventional adenomas.120,121 Therefore, group 3 CRC may arise within mixed polyps or serrated adenomas as well as conventional adenomas with villous change.120 p r e c u r s or l e s i o n s m uc i n o u s d i f f e r e n t i a t ion and d irty necrosis Lesions similar to hyperplastic polyps but characterized by large size, aberrant architecture, increased proliferation and a predilection for the proximal colon have recently been linked with sporadic MSI-H CRC and termed ‘sessile serrated adenoma (SSA)’,112,113 ‘sessile serrated polyp’22 or ‘sessile polyp with atypical proliferation’ (Figure 2c).53,114 The finding of either this lesion or traditional serrated adenoma in contiguity with a CRC would serve as evidence of molecular groups 1 or 2.82,115,116 Most of the polyps contiguous with CRC in Lynch syndrome are conventional adenomas,115,117 but serrated adenoma has been observed on rare occasions.118 The transition from SSA to CRC will usually be through an intermediate stage of dysplasia or intraepithelial neoplasia (giving a mixed polyp), even if this step is transient. Importantly, dysplasia in the serrated pathway may not resemble adenomatous dysplasia. Instead of being elongated, pseudo-stratified and hyperchromatic, nuclei are round and vesicular with a coarse nuclear membrane and a prominent nucleolus and nuclear polarity is well maintained.119,120 In other words, the cytological atypia resembles (not surprisingly) the aberrant cytology associated with group 1 and 2 CRC as described above. Molecular alterations occurring at the key transition from hyperplasia to dysplasia include loss of expression of MLH1 in group 1 CRC and loss of expression of MGMT and ⁄ or aberrant expression of p53 in group 2 CRC (Figure 2d–f). Mixed polyps, in which the dysplastic component shows normal expression of MLH1 but aberrant expression of p53, have been termed ‘fusion’ polyps since they combine molecular features of the serrated pathway (e.g. BRAF mutation and DNA methylation) with an abnormality characteristic of the adenoma–carcinoma sequence.120 Therefore, group 2 CRC could be regarded as a group 1 ⁄ group 4 hybrid. Group 3 CRC is associated with KRAS mutation and CIMP-low (CIMP2) (see above). The principal precursors of group 3 CRC are likely to be adenomas with KRAS mutation. DNA methylation occurs in adenomas but becomes more evident with increasing size, dysplasia or villosity.49–51 However, there is less extensive marker methylation in adenomas Mucinous carcinoma is diagnosed when at least 50% of the tumour comprises secretory mucin. The mucin is intraluminal in the case of well or moderately differentiated CRC and forms interstitial pools surrounding the irregular trabeculae in poorly differentiated CRC (Figure 3a,b).122 Mucinous carcinoma is over-represented among group 1 and 2 CRC61–63,65,66,123 and the latter may secrete appreciable amounts of mucin without meeting the strict quantitative definition. In the case of group 1 (sporadic MSI-H) CRC there is often a zoning pattern with mucin secretion confined to the deeper tumour compartment only,124 or there may be marked tumour heterogeneity with areas of mucinous carcinoma alternating with other patterns.125 The strict definition of mucinous carcinoma may therefore lack sensitivity when it is used as a marker of group 1 and 2 CRC. Secretory mucin associated with group 1 (sporadic MSI-H) CRC comprises both intestinal (MUC2) and gastric (MUC5AC) mucin.123,126 Secretory mucin associated with serrated adenocarcinomas comprises non-O-acetylated sialic acid substituents, as in small intestine.109 A mixed gastric and small intestinal mucinous phenotype is also associated with hyperplastic polyps, mixed polyps and serrated adenomas of the colorectum.109,127 By contrast, secretory mucin production in conventional colorectal adenomas is decreased, leaving expression of the transmembrane glycoprotein MUC1 only in areas of high-grade dysplasia.128 Expression of non-O-acetylated sialic acid is also restricted to foci of high-grade dysplasia in adenomas.129 A shared secretory mucinous profile across serrated polyps, group 1 (sporadic MSI-H) CRC and serrated adenocarcinoma (occurring among group 1 and 2 CRC) provides strong evidence for the existence of a serrated pathway of colorectal tumorigenesis which parallels the molecular arguments presented above. Some sporadic mucinous carcinomas arise in villous adenomas (Figure 3b).130 The increased frequency of villous adenomas in Lynch syndrome7 may account for the higher incidence of mucinous carcinoma in this condition.131,132 The link between mucinous differentiation and Lynch syndrome was observed in CRC 2007 The Author. Journal compilation 2007 Blackwell Publishing Ltd, Histopathology, 50, 113–130. J R Jass 122 a b c d e f Figure 3. Differentiation, tumour budding and lymphocytic infiltration. Mucinous carcinoma with serrated morphology (group 1) (a) is compared with mucinous carcinoma (group 3 or 4) that developed within a villous adenoma (b). In addition to the serrated contour of epithelium, the mucinous carcinoma arising within a serrated precursor lesion (not shown) is characterized by an abundant eosinophilic cytoplasm and vesicular ovoid nuclei that are ovoid and vesicular with a prominent nucleolus (a). The cytology of the mucinous carcinoma arising in a villous adenoma (not shown) is characterized by a dark and amphophilic cytoplasm and nuclei which are hyperchromatic rather than vesicular (b). Moderately differentiated adenocarcinoma (group 4), in which lumen contains necrotic cellular debris (‘dirty necrosis’) and epithelium shows elongated and stratified nuclei which are hyperchromatic and lack distinct nucleoli (c). The cytology is consistent with an origin in a conventional adenoma and not a serrated polyp. Medullary carcinoma (group 5, Lynch syndrome) composed of solid sheets of cells and infiltrated by lymphocytes (arrows) (d). Tumour budding characterized by small clusters of de-differentiated cells (arrow) at the invasive margin (e). Moderately differentiated adenocarcinoma (group 5, Lynch syndrome) with intraepithelial lymphocytes (arrows) (f). The cytology is consistent with origin within a conventional adenoma. 2007 The Author. Journal compilation 2007 Blackwell Publishing Ltd, Histopathology, 50, 113–130. Clasification of colorectal cancer obtained from subjects meeting clinical criteria for Lynch syndrome and before its molecular basis had been uncovered. Following the demonstration of DNA mismatch repair deficiency and the associated MSI phenotype, sporadic and familial CRCs with MSI-H were initially grouped together on the assumption that they were equivalent tumours.124,133 However, a marked mucinous component has been described in 35%,133 43%,125 36%134 and 31%135 of MSI-H CRCs that were mainly sporadic. When the mucinous phenotype was defined on the basis of any amount of secretory mucin, this feature was found in as many as 67% of mainly sporadic MSI-H CRC.136 In contrast, mucinous differentiation was observed in only 19%132 and 22%125 of likely Lynch syndrome CRC, whereas, among 64 CRCs from subjects with a proven germ-line mutation in a DNA mismatch repair gene, the frequency of mucinous carcinoma was not significantly greater than in CRC from in general population.137 It is likely that there is a slight over-representation of mucinous carcinoma in Lynch syndrome, but with interfamily differences. Mucinous carcinoma has been reported in five members of a single family138 and has also been associated specifically with MSH2 germ-line mutation.137 Mucinous carcinoma has traditionally been regarded as relatively aggressive, although this impression derives mainly from the study of rectal cancer.130,139 It is clear that mucinous differentiation occurs in multiple molecular subtypes, each with differing site predilections and prognosis. Since mucinous differentiation is not specific to a single clinicopathological entity, the lack of a clear prognostic effect is not surprising. When not filled with secretory mucin, malignant lumina in haematoxylin and eosin (H&E) sections may either appear empty or contain deeply eosinophilic material that is frequently admixed with necrotic cell debris (Figure 3c). This eosinophilic material (‘dirty necrosis’) is strongly positive with period acid–Schiff and expresses the transmembrane glycoprotein MUC1.140 The presence of dirty necrosis is negatively associated with CRC showing MSI-H.136 p o or d i f f e r e n t i a t i o n , m e d u l l a ry a n d s i gn e t ring cell subtypes Poor differentiation, as in other tumour types, indicates a marked loss of morphological resemblance to the parent tissue and, in the case of adenocarcinoma, a loss of glandular development. Like mucinous differentiation, this feature has been associated with poor prognosis. However, and serving as a parallel with mucinous differentiation, poorly differentiated adenocarcinoma is over-represented among group 1141 and 123 Lynch syndrome CRC131,132 that are associated with a relatively good prognosis. A series of eight largely undifferentiated CRCs with a pushing tumour margin was found to be associated with an unexpectedly favourable clinical outcome.142 Two of the subjects were very young (a female aged 31 years and a male aged 39 years) and may well have had Lynch syndrome. The term medullary carcinoma has subsequently been applied to poorly differentiated large cell carcinoma in which the epithelium is arranged in closely packed trabeculae or solid aggregates.143 Medullary carcinoma is distinguished from undifferentiated carcinoma by its good overall circumscription, lack of nuclear pleomorphism, presence of lymphocytic infiltration, which may be intraepithelial, peritumoral or within Crohn-like nodules, and presence of focal glandular differentiation (Figure 3d). Medullary carcinoma occurs in both group 1 and Lynch syndrome CRC but is uncommon in both. However, Group 1 CRCs frequently show morphological heterogeneity with the medullary pattern being represented in subclones.125 The homeobox gene CDX2 is mutated in CRC with MSI-H144 but (and consistent with the rarity of medullary carcinoma) mutation of this gene was observed in only 3.2% of Lynch syndrome CRC.145 Loss of expression of CDX2 was strongly associated with medullary carcinoma (described as large cell minimally differentiated carcinoma).146 In grading the biological aggressiveness of CRC it is clear that a single feature, such as glandular differentiation, provides limited prognostic information when assessed in isolation from other features, whether morphological or molecular. Although associated with group 1 and Lynch syndrome CRC, signet ring cell carcinoma is, like medullary carcinoma, an uncommon malignancy and is probably less specific than medullary carcinoma with respect to an association with MSI. Due to its relative rarity it is not known if the highly aggressive nature of signet ring cell carcinoma is modified in CRC with MSI-H status. This possibility is, however, suggested by the description of well-circumscribed signet ring cell carcinomas with an exophytic growth pattern and limited aggression.147 CRCs with both CIMP and MSI-L status (group 2) are more likely to include subclones comprising signet ring cells.148 i n v a s i v e m a r g i n a n d t u m ou r b u d di n g Morphological findings at the invasive tumour margin provide, after stage, the most important prognostic information in CRC.149–151 A diffusely infiltrative margin is characterized by widespread invasion and dissection of normal tissue structures (smooth muscle or 2007 The Author. Journal compilation 2007 Blackwell Publishing Ltd, Histopathology, 50, 113–130. 124 J R Jass adipose tissue) so that there is no clear boundary between tumour and host tissue. This pattern of spread is closely correlated with lymphovascular and perineural invasion and the presence of discontinuous mesenteric deposits.152 A diffuse growth pattern is also associated with a feature described as tumour budding or de-differentiation, in which there is a transition from glandular structures to single cells or clusters of up to four cells at the invasive margin (Figure 3e).151 Tumour budding may and should be distinguished from a subclone showing poor differentiation by its presence along the entire invasive interface. Furthermore, budding cells have the properties of malignant stem cells, including the potential for re-differentiation both locally and at sites of metastasis.153 In other words, the morphological and immunophenotypic features associated with budding cells are reversible and therefore likely to be under epigenetic control. Expression patterns associated with budding cells include upregulation of b-catenin,154,155 laminin5-c2,156 matrix metalloproteinase-7 (MMP-7 or matrilysin),157 membrane type-1 MMP,157 p16,28 cyclin D1,33 urokinase-like plasminogen activator receptor,158 158 158 159 CD44, COX-2 and tenascin-C and down-regulation of E-cadherin155,160 and Cdx-2.161 Budding cells also show evidence of autonomous movement characterized by the presence of podia162 that express P-glycoprotein at points of attachment to mesenchymal elements.163 Mesenchymal markers including fibronectin are also expressed155 and tumour budding is synonymous with the epithelial–mesenchymal transition described in other cancer model systems.159 While tumour budding is likely to be triggered through an increased sensitivity to mesenchymally derived growth signals,161 the change will occur only in cancer cells primed by particular genetic alterations. Tumour budding is uncommon in group 1 CRC28,164 and when it does occur the full immunophenotype is not apparent.165 For example, budding cells in group 1 CRC do not show increased expression of b-catenin or laminin5-c2 and lack the development of podia.165 It is possible that the low frequency of mutation of the Wnt pathway genes APC and CTNNB1 accounts for the lack of tumour budding in group 1 CRC. While the full budding phenotype may not be an absolute requirement for metastasis, the relative absence of budding among group 1 CRCs could be at least one explanation for their good prognosis. lymphocytic infiltration A marked peritumoral lymphocytic infiltrate was initially described in CRC from subjects meeting clinical criteria for Lynch syndrome.132 Intraepithelial lymphocytes, known also as TILs, were initially associated with undifferentiated or medullary carcinoma with MSI-H,166 but were subsequently also shown to be a useful biomarker for all group 1 (sporadic MSI-H) CRC.141 TILs serve as the most important marker for sporadic and familial MSI-H CRC and diagnostic cut-offs based on cell counts in either H&E sections136,167 or utilizing CD3 ⁄ CD8 immunohistochemistry124,168 have been established. The finding of at least five intraepithelial lymphocytes in at least one of 10 high-power (· 40) fields provides a sensitive cut-off (Figure 3f). Intraepithelial cytotoxic (CD8) T cells are observed under normal physiological conditions. Accordingly, one might postulate a mechanism leading to active destruction of these cells in non-MSI-H CRC, for example by the ‘Fas ligand counter-attack’.169 However, intraepithelial T cells in MSI-H CRC are: (i) generally more numerous than under normal physiological conditions, (ii) associated with a peritumoral lymphocytic reaction including Crohn-like nodules of B cells170 and (iii) associated with an improved prognosis within the MSI-H subset.171 These findings indicate the existence of a clinically beneficial specific immune reaction against mutator-generated tumour antigens and not merely the passive retention of T cells within the intraepithelial compartment. There is a negative correlation between lymphocytic infiltration and mucinous differentiation139 and this explains why these features are independent markers of MSI-H status. Lymphocytic infiltration (peritumoral and Crohn-like) was more marked in Lynch syndrome than group 1 (sporadic MSI-H) CRC,115 a finding which could be related to the increased frequency of mucinous differentiation in the latter (see above). It is also possible that the adverse prognosis associated with TIL-depleted MSI-H CRC is explained by the deleterious effects of increased mucin production. TILs are not restricted to the MSI-H subset of CRC. Increased TIL counts have been associated with MSI-L status168 and particularly in CRC with both CIMP-high and MSI-L (group 2).148 Since this subset is not associated with a good prognosis, the link between TILs and survival remains unclear. Conclusion A classification of CRC that incorporates an understanding of the earliest evolutionary steps is necessary in order to dissect out the various risk factors that explain causation or pathogenesis or identify early targets for chemoprevention. 2007 The Author. Journal compilation 2007 Blackwell Publishing Ltd, Histopathology, 50, 113–130. Clasification of colorectal cancer The notion that adenomas give rise to CRC was developed by pathologists. It was subsequently re-worked by basic scientists, who promulgated the view that adenomas are initiated through bi-allelic inactivation of APC and progress to CRC through a predictable linear sequence of molecular alterations. The dogmatic linking of the ‘vast majority’ of CRC to this mono-directional model implied that if a minority subset outside the model existed it was too minuscule to warrant further consideration. Until comparatively recently there has been a failure to recognize that CRC is in fact a multipathway disease comprising disparate subgroups with particular clinical, pathological and molecular features. The unfortunate consequence has been a delay in the progress of research that depends absolutely on such an understanding. In particular, the oversimplification of the evolutionary pathway has confounded the identification of risk factors for CRC, whether genetic, constitutional or lifestyle related. While it has been usual to establish the molecular correlates of existing morphological classifications, the decades-long tendency of considering CRC as a single entity means that the circle has had to be completed in the reverse order. It should be stressed, however, that the correlation of morphological and immunohistochemical features with molecular subtypes has been an iterative process, in which genetic instability and CIMP have been shown to be fundamental classification criteria through a process of trial and error. This correlative process incorporates clinical, morphological and biological components. Furthermore, since genetic instability and CIMP are acquired at the precancerous stage, the suggested typing of CRC has a strong basis in pathogenesis. While the proposed classification remains speculative, it has the advantage of conveying powerful meaning through the synthesis of clinical, pathological and molecular features. An exclusively molecular classification carries little meaning. John Constable said that we see nothing until we truly understand, but we can also say that we understand nothing until we truly see. The recognition of the heterogeneous nature of CRC means that pathology has now become integral to CRC research. References 1. Iacopetta B. Are there two sides to colorectal cancer? Int. J. Cancer 2002; 101; 403–408. 2. Vogelstein B, Fearon ER, Hamilton SR et al. Genetic alterations during colorectal-tumor development. N. Engl. J. Med. 1988; 319; 525–532. 3. Fodde R, Kuipers J, Rosenberg C et al. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat. Cell Biol. 2001; 3; 433–438. 125 4. Tomlinson I, Bodmer W. Selection, the mutation rate and cancer: ensuring that the tail does not wag the dog. Nat. Med. 1999; 5; 11–12. 5. Nowell PC. The clonal evolution of tumor cell populations. Science 1976; 194; 23–28. 6. Eide TJ. Risk of colorectal cancer in adenoma-bearing individuals within a defined population. Int. J. Cancer 1986; 38; 173–176. 7. Jass JR, Stewart SM. Evolution of hereditary non-polyposis colorectal cancer. Gut 1992; 33; 783–786. 8. Iino H, Simms LA, Young J et al. DNA microsatellite instability and mismatch repair protein loss in adenomas presenting in hereditary non-polyposis colorectal cancer. Gut 2000; 47; 37–42. 9. Markowitz S, Wang J, Myeroff L et al. Inactivation of the type II TGF-b receptor in colon cancer cells with microsatellite instability. Science 1995; 268; 1336–1338. 10. Souza RF, Appel R, Yin J et al. The insulin-like growth factor II receptor gene is a target of microsatellite instability in human gastrointestinal tumours. Nat. Genet. 1996; 14; 255–257. 11. Rampino N, Yamamoto H, Ionov Y et al. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science 1997; 275; 967–969. 12. Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993; 363; 558–561. 13. Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell 1996; 87; 159–170. 14. Aaltonen LA, Peltomaki PS, Leach FS et al. Clues to the pathogenesis of familial colorectal cancer. Science 1993; 260; 812–816. 15. Huang J, Papadopoulos N, McKinley AJ et al. APC mutations in colorectal tumors with mismatch repair deficiency. Proc. Natl Acad. Sci. USA 1996; 93; 9049–9054. 16. Konishi M, Kikuchi-Yanoshita R, Tanaka K et al. Molecular nature of colon tumors in hereditary nonpolyposis colon cancer, familial polyposis, and sporadic colon cancer. Gastroenterology 1996; 111; 307–317. 17. Losi L, Ponz de Leon M, Jiricny J et al. K-ras and p53 mutations in hereditary non-polyposis colorectal cancers. Int. J. Cancer 1997; 74; 94–96. 18. Tsuchiya A, Nomizu T, Onda M, Ohki S, Sato H, Abe R. Molecular genetic alteration and DNA ploidy in hereditary nonpolyposis colorectal cancer. Int. J. Clin. Oncol. 1997; 2; 224–229. 19. Fujiwara T, Stoker JM, Watanabe T et al. Accumulated clonal genetic alterations in familial and sporadic colorectal carcinomas with widespread instability in microsatellite sequences. Am. J. Pathol. 1998; 153; 1063–1078. 20. Tomlinson I, Ilyas M, Johnson V et al. A comparison of the genetic pathways involved in the pathogenesis of three types of colorectal cancer. J. Pathol. 1998; 184; 148–152. 21. Grady WM, Rajput A, Myeroff L et al. Mutation of the type II transforming growth factor-b receptor is coincident with the transformation of human colon adenomas to malignant carcinomas. Cancer Res. 1998; 58; 3101–3104. 22. Jass JR. Hyperplastic-like polyps as precursors of microsatellite unstable colorectal cancer. Am. J. Clin. Pathol. 2003; 119; 773–775. 23. Jass JR. Towards a molecular classification of colorectal cancer. Int. J. Colorectal Dis. 1999; 14; 194–200. 24. Young J, Leggett B, Gustafson C et al. Genomic instability occurs in colorectal carcinomas but not in adenomas. Hum. Mutat. 1993; 2; 351–354. 2007 The Author. Journal compilation 2007 Blackwell Publishing Ltd, Histopathology, 50, 113–130. 126 J R Jass 25. Loukola A, Salovaara R, Kristo P et al. Microsatellite instability in adenomas as a marker for hereditary nonpolyposis colorectal cancer. Am. J. Pathol. 1999; 155; 1849–1853. 26. Salahshor S, Kressner U, Påhlman L, Glimelius B, Lindmark G, Lindblom A. Colorectal cancer with and without microsatellite instability involves different genes. Genes Chromosomes Cancer 1999; 26; 247–252. 27. Jass JR, Biden KG, Cummings M et al. Characterisation of a subtype of colorectal cancer combining features of the suppressor and mild mutator pathways. J. Clin. Pathol. 1999; 52; 455–460. 28. Jass JR, Barker M, Fraser L et al. APC mutation and tumour budding in colorectal cancer. J. Clin. Pathol. 2003; 56; 69–73. 29. Mirabelli-Primdahl L, Gryfe R, Kim H et al. Beta-catenin mutations are specific for colorectal carcinomas with microsatellite instability but occur in endometrial carcinomas irrespective of mutator pathway. Cancer Res. 1999; 59; 3346–3351. 30. Miyaki M, Iijima T, Kimura J et al. Frequent mutation of b-Catenin and APC genes in primary colorectal tumors from patients with hereditary nonpolyposis colorectal cancer. Cancer Res. 1999; 59; 4506–4509. 31. Johnson V, Volikos E, Halford SER et al. Exon 3 beta-catenin mutations are specifically associated with colorectal carcinomas in the hereditary non-polyposis colorectal cancer syndrome. Gut 2004; 53; 264–267. 32. Samowitz WS, Powers MD, Spirio LN, Nollet F, van Roy F, Slattery ML. b-catenin mutations are more frequent in small colorectal adenomas than in larger adenomas and invasive carcinomas. Cancer Res. 1999; 59; 1442–1444. 33. Wong NACS, Morris RG, McCondochie A, Bader S, Jodrell DI, Harrison DJ. Cyclin D1 overexpression in colorectal carcinoma in vivo is dependent on b-catenin protein dysregulation, but not k-ras mutation. J. Pathol. 2002; 197; 128–135. 34. Shitoh K, Furukawa T, Kojima M et al. Frequent activation of the beta-catenin–Tcf signalling pathway in nonfamilial colorectal cancer with microsatellite instability. Genes Chromosomes Cancer 2001; 30; 32–37. 35. Koinuma K, Shitoh K, Miyakura Y et al. Mutations of BRAF are associated with extensive hMLH1 promoter methylation in sporadic colorectal carcinomas. Int. J. Cancer 2004; 108; 237– 242. 36. Gazzoli I, Loda M, Garber J, Syngal S, Kolodner RD. A hereditary nonpolyposis colorectal carcinoma case associated with hypermethylation of the hMLH1 gene in normal tissue and loss of heterozygosity of the unmethylated allele in the resulting microsatellite instability-high tumor. Cancer Res. 2002; 62; 3925–3928. 37. Sparks AB, Morin PJ, Vogelstein B, Kinzler KW. Mutational analysis of the APC ⁄ beta-catenin ⁄ Tcf pathway in colorectal cancer. Cancer Res. 1998; 58; 1130–1134. 38. Esteller M, Sparks A, Toyota M et al. Analysis of adenomatous polyposis coli promoter hypermethylation in human cancer. Cancer Res. 2000; 60; 4366–4371. 39. Schneikert J, Behrens J. Truncated APC is required for cell proliferation and DNA replication. Int. J. Cancer 2006; 119; 74–79. 40. Liu W, Dong X, Mai M et al. Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating b-catenin ⁄ TCF signalling. Nat. Genet 2000; 26; 146–147. 41. Thorstensen L, Lind GE, Lovig T et al. Genetic and epigenetic changes of components affecting the WNT pathway in colorectal carcinomas stratified by microsatellite instability. Neoplasia 2005; 7; 99–108. 42. Chan TL, Zhao W, Leung SY, Yuen ST. BRAF and KRAS mutations in colorectal hyperplastic polyps and serrated adenomas. Cancer Res. 2003; 63; 4878–4881. 43. Konishi K, Yamochi T, Makino R et al. Molecular differences between serrated and conventional colorectal adenomas. Clin. Cancer Res. 2004; 10; 3082–3090. 44. Kambara T, Simms LA, Whitehall VLJ et al. BRAF mutation and CpG island methylation: an alternative pathway to colorectal cancer. Gut 2004; 53; 1137–1144. 45. Ikehara N, Semba S, Sakashita M, Aoyama N, Kasuga M, Yokozaki H. BRAF mutation associated with dysregulation of apoptosis in human colorectal neoplasms. Int. J. Cancer 2005; 115; 943–950. 46. Wang L, Cunningham JM, Winters JL et al. BRAF mutations in colon cancer are not likely attributable to defective DNA mismatch repair. Cancer Res. 2003; 63; 5209–5212. 47. McGivern A, Wynter CVA, Whitehall VLJ et al. Promoter hypermethylation frequency and BRAF mutations distinguish hereditary non-polyposis colon cancer from sporadic MSI-H colon cancer. Familial Cancer 2004; 3; 101–107. 48. Weisenberger DJ, Siegmund KD, Campan M et al. A distinct CpG island methylator phenotype in human colorectal cancer is the underlying cause of sporadic mismatch repair deficiency and is tightly associated with BRAF mutation. Nat. Genet. 2006; 38; 787–793. 49. Rashid A, Shen L, Morris JS, Issa J-PJ, Hamilton SR. CpG island methylation in colorectal adenomas. Am. J. Pathol. 2001; 159; 1129–1135. 50. Wynter CVA, Kambara T, Walsh MD, Leggett BA, Young J, Jass JR. DNA methylation patterns in adenomas from FAP, multiple adenoma and sporadic carcinoma patients. Int. J. Cancer 2006; 118; 907–915. 51. Kim HC, Roh SA, Ga IK, Kim JS, Yu CS, Kim JC. CpG island methylation as an early event during adenoma progression in carcinogenesis of sporadic colorectal cancer. J. Gastroenterol. Hepatol. 2005; 20; 1920–1926. 52. Wynter CV, Walsh MD, Higuchi T, Leggett BA, Young J, Jass JR. Methylation patterns define two types of hyperplastic polyp associated with colorectal cancer. Gut 2004; 53; 573–580. 53. Yang S, Farraye FA, Mack C, Posnik O, O’Brien MJ. BRAF and KRAS mutations in hyperplastic polyps and serrated adenomas of the colorectum: relationship to histology and CpG island methylation status. Am. J. Surg. Pathol. 2004; 28; 1452–1459. 54. Iino H, Jass JR, Simms LA et al. DNA microsatellite instability in hyperplastic polyps, serrated adenomas, and mixed polyps: a mild mutator pathway for colorectal cancer? J. Clin. Pathol. 1999; 52; 5–9. 55. Jass JR, Iino H, Ruszkiewicz A et al. Neoplastic progression occurs through mutator pathways in hyperplastic polyposis of the colorectum. Gut 2000; 47; 43–49. 56. Mäkinen MJ, George SMC, Jernvall P, Mäkelä J, Vihko P, Karttunen TJ. Colorectal carcinoma associated with serrated adenoma—prevalence, histological features, and prognosis. J. Pathol. 2001; 193; 286–294. 57. Oh K, Redston M, Odze RD. Support for hMLH1 and MGMT silencing as a mechanism of tumorigenesis in the hyperplastic– adenoma–carcinoma (serrated) carcinogenic pathway in the colon. Hum. Pathol. 2005; 36; 101–111. 58. Kane MF, Loda M, Gaida GM et al. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 1997; 57; 808–811. 2007 The Author. Journal compilation 2007 Blackwell Publishing Ltd, Histopathology, 50, 113–130. Clasification of colorectal cancer 59. Smith G, Carey FA, Beattie J et al. Mutations in APC, Kirstenras, and p53—alternative genetic pathways to colorectal cancer. Proc. Natl Acad. Sci. USA 2002; 99; 9433–9438. 60. Frattini D, Balestra D, Suardi S et al. Different genetic features associated with colon and rectal carcinogenesis. Clin. Cancer Res. 2004; 10; 4015–4021. 61. Samowitz WS, Sweeney C, Herrick J et al. Poor survival associated with the BRAF V600E mutation in microsatellitestable colon cancers. Cancer Res. 2005; 65; 6063–6069. 62. Whitehall VLJ, Wynter CVA, Walsh MD et al. Morphological and molecular heterogeneity within non-microsatellite instability-high colorectal cancer. Cancer Res. 2002; 62; 6011– 6014. 63. Hawkins N, Norrie M, Cheong K et al. CpG island methylation in sporadic colorectal cancer and its relationship to microsatellite instability. Gastroenterology 2002; 122; 1376–1387. 64. van Rijnsoever M, Elsaleh H, Joseph D, McCaul K, Iacopetta B. CpG island methylator phenotype is an independent predictor of survival benefit from 5-fluorouracil in stage III colorectal cancer. Clin. Cancer Res. 2003; 9; 2898–2903. 65. Chirieac LR, Shen L, Catalano PJ, Issa J-P, Hamilton SR. Phenotype of microsatellite-stable colorectal carcinomas with CpG island methylation. Am. J. Surg. Pathol. 2005; 29; 429– 436. 66. Samowitz WS, Albertsen H, Herrick J et al. Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology 2005; 129; 837–845. 67. Georgiades IB, Curtis LJ, Morris RM, Bird CC, Wyllie AH. Heterogeneity studies identify a subset of sporadic colorectal cancers without evidence for chromosomal or microsatellite instability. Oncogene 1999; 18; 7933–7940. 68. Hawkins NJ, Tomlinson I, Meagher A, Ward RL. Microsatellitestable diploid carcinoma: a biologically distinct and aggressive subset of colorectal cancer. Br. J. Cancer 2001; 84; 232–236. 69. Goel A, Arnold CN, Niedzwiecki D et al. Characterization of sporadic colon cancer by patterns of genomic instability. Cancer Res. 2003; 63; 1608–1614. 70. Tang R, Changchien CR, Wu M-C et al. Colorectal cancer without high microsatellite instability and chromosomal instability—an alternative genetic pathway to human colorectal cancer. Carcinogenesis 2004; 25; 841–846. 71. Sugai T, Habano W, Jiao Y-F et al. Analysis of molecular alterations in left- and right-sided colorectal carcinomas reveals distinct pathways of carcinogenesis. Proposal for new molecular profile of colorectal carcinomas. J. Mol. Diagn. 2006; 8; 193–201. 72. Ward RL, Cheon K, Ku S-U, Meagher A, O’Connor T, Hawkins NJ. Adverse prognostic effect of methylation in colorectal cancer is reversed by microsatellite instability. J. Clin. Oncol. 2003; 21; 3729–3736. 73. Halford S, Sasieni P, Rowan A et al. Low-level microsatellite instability occurs in most colorectal cancers and is a nonrandomly distributed quantitative trait. Cancer Res. 2002; 62; 53–57. 74. Halford SER, Sawyer EJ, Lambros MB et al. MSI-low, a real phenomenon which varies in frequency among cancer types. J. Pathol. 2003; 201; 389–394. 75. Mori Y, Selaru FM, Sato F et al. The impact of microsatellite instability in the molecular phenotype of colorectal tumors. Cancer Res. 2003; 63; 4577–4582. 76. Iglesias D, Fernandez-Peralta AM, Nejda N et al. RIS1, a gene with trinucleotide repeats, is a target in the mutator pathway 77. 78. 79. 80. 81. 82. 83. 84. 85. 86. 87. 88. 89. 90. 91. 92. 93. 94. 127 of colorectal carcinogenesis. Cancer Genet. Cytogenet. 2006; 167; 138–144. Wright CM, Dent OF, Newland RC et al. Low level microsatellite instability may be associated with reduced cancer specific survival in sporadic stage C colorectal cancer. Gut 2005; 54; 103–108. Kohonen-Corish MRJ, Daniel JJ, Chan C et al. Low microsatellite instability is associated with poor prognosis in stage C colon cancer. J. Clin. Oncol. 2005; 23; 2318–2324. Ogino S, Cantor M, Kawasaki T et al. CpG island methylator phenotype (CIMP) of colorectal cancer is best characterized by quantitative DNA methylation analysis and prospective cohort studies. Gut 2006; 55; 1000–1006. Nagasaka T, Sasamoto H, Notohara K et al. Colorectal cancer with mutation in BRAF, KRAS, and wild-type with respect to both oncogenes showing differing patterns of DNA methylation. J. Clin. Oncol. 2004; 22; 4584–4594. Whitehall VLJ, Walsh MD, Young J, Leggett BA, Jass JR. Methylation of 0-6-Methylguanine DNA Methyltransferase characterises a subset of colorectal cancer with low level DNA microsatellite instability. Cancer Res. 2001; 61; 827–830. Tuppurainen K, Makinen JM, Junttila O et al. Morphology and microsatellite instability in sporadic serrated and non-serrated colorectal cancer. J. Pathol. 2005; 207; 285–294. Mahooti S, Hampel H, LaJeunesse J, Sotamaa K, de la Chapelle A, Frankel WL. MLH1 and PMS2 protein expression in 103 colorectal carcinomas with MLH1 promoter methylation and without MLH1 or PMS2 germline mutation. Lab. Invest. 2006; 86 (Suppl 1); 113A. Esteller M, Toyota M, Sanchez-Cespedes M et al. Inactivation of the DNA repair gene 06-Methylguanine-DNA Methyltransferase by promoter hypermethylation is associated with G to A mutations in K-ras in colorectal tumorigenesis. Cancer Res. 2000; 60; 2368–2371. Fink D, Aebi S, Howell SB. The role of DNA mismatch repair in drug resistance. Clin. Cancer Res. 1998; 4; 1–6. Branch P, Aquilina G, Bignami M, Karran P. Defective mismatch binding and a mutator phenotype in cells tolerant to DNA damage. Nature 1993; 362; 652–654. Issa J-P. CIMP, at last. Gastroenterology 2005; 129; 1121–1124. Shen L, Toyota M, Kondo Y et al. Two distinct DNA methylator phenotypes in colorectal cancer. Proc. Am. Assoc Cancer Res. 2006; Abst. 5727. Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997; 88; 593–602. Michaloglou C, Vredeveld CW, Soengas MS et al. BRAFE600associated senescence-like cell cycle arrest of human naevi. Nature 2005; 436; 720–724. Kaye GE, Fenoglio CM, Pascal RR, Lane N. Comparative electron microscopic features of normal, hyperplastic and adenomatous human colonic epithelium. Gastroenterology 1973; 64; 926–945. Hayashi T, Yatani R, Apostol J, Stemmermann GN. Pathogenesis of hyperplastic polyps of the colon: a hypothesis based on ultrastructure and in vitro cell kinetics. Gastroenterology 1974; 66; 347–356. Minoo P, Baker K, Goswami R et al. Extensive DNA methylation in normal colorectal mucosa in hyperplastic polyposis. Gut 2006; 55; 1467–1474. Keshet I, Schlesinger Y, Frakash S et al. Evidence for an instructive mechanism of de novo methylation in cancer cells. Nat. Genet. 2006; 38; 149–153. 2007 The Author. Journal compilation 2007 Blackwell Publishing Ltd, Histopathology, 50, 113–130. 128 J R Jass 95. Frazier ML, Xi L, Zong J et al. Association of the CpG island methylator phenotype with family history of cancer in patients with colorectal cancer. Cancer Res. 2003; 63; 4805–4808. 96. Vandrovcova J, Lagerstedt-Robinsson K, Lindblom A. BRAF mutations suggest a new familial colorectal cancer syndrome. Proc. Am. Assoc. Cancer Res. 2006; Abst. 2972. 97. Young J, Barker MA, Simms LA et al. BRAF mutation and variable levels of microsatellite instability characterize a syndrome of familial colorectal cancer. Clin. Gastroenterol. Hepatol. 2005; 3; 254–263. 98. Ward RL, Williams R, Law M, Hawkins NJ. The CpG island methylator phenotype is not associated with a personal or family history of cancer. Cancer Res. 2004; 64; 7618–7621. 99. Samowitz WS, Sweeney C, Albertsen H, Wolff RK, Slattery ML. Smoking is asociated with the CpG island methylator phenotype and V600E BRAF mutations in colon cancer. Proc. Am. Assoc. Cancer Res. 2006; Abst. 3709. 100. Morimoto LM, Newcomb PA, Ulrich CM, Bostick RM, Lais CJ, Potter JD. Risk factors for hyperplastic and adenomatous polyps: evidence for malignant potential. Cancer Epidemiol. Biomark Prev. 2002; 11; 1012–1018. 101. Yu J-H, Bigler J, Whitton J, Potter JD, Ulrich CM. Mismatch repair polymorphisms and colorectal polyps: hMLH1–93G>A variant modifies risk associated with smoking. Am. J. Gastroenterol. 2006; 101; 1313–1319. 102. Issa J-P, Ahuja N, Toyota M, Bronner MP, Brentnall TA. Accelerated age-related CpG island methylation in ulcerative colitis. Cancer Res. 2001; 61; 3573–3577. 103. Cui H, Horon IL, Ohlsson R, Hamilton SR, Feinberg AP. Loss of imprinting in normal tissue of colorectal cancer patients with microsatellite instability. Nat. Med. 1998; 4; 1276–1280. 104. Sasaki J-I, Konishi F, Kawamura YJ, Kai T, Takata O, Tsukamoto T. Clinicopathological characteristics of colorectal cancers with loss of imprinting of insulin-like growth factor 2. Int. J. Cancer 2006; 119; 80–83. 105. Nakagawa H, Chadwick RB, Peltomaki P, Plass C, Nakamura Y, de la Chapelle A. Loss of imprinting of the insulin-like growth factor II gene occurs by bi-allelic methylation in a core region of H19-associated CTCF-binding sites in colorectal cancer. Proc. Natl Acad. Sci. USA 2001; 98; 591–596. 106. Cui H, Cruz-Correa M, Giardiello FM et al. Loss of IGF2 imprinting: a potential marker of colorectal cancer risk. Science 2003; 299; 1753–1755. 107. Kruhoffer M, Jensen JL, Laiho P et al. Gene expression signatures for colorectal cancer microsatellite status and HNPCC. Br. J. Cancer 2005; 92; 2240–2248. 108. Al-Tassan N, Chmiel NH, Maynard J et al. Inherited variants of MYH asociated with somatic G:C–T:A mutations in colorectal tumors. Nat. Genet. 2002; 30; 227–232. 109. Jass JR, Smith M. Sialic acid and epithelial differentiation in colorectal polyps and cancer—a morphological, mucin and lectin histochemical study. Pathology 1992; 24; 233–242. 110. Yao T, Nishiyama K-I, Oya M, Kouziki T, Kajiwara M, Tsuneyoshi M. Multiple ‘serrated adenocarcinomas’ of the colon with a cell lineage common to metaplastic polyp and serrated adenoma. Case report of a new subtype of colonic adenocarcinoma with gastric differentiation. J. Pathol. 2000; 190; 444–449. 111. Jass JR. HNPCC and sporadic MSI-H colorectal cancer: a review of the morphological similarities and differences. Familial Cancer 2004; 3; 93–100. 112. Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am. J. Surg. Pathol. 2003; 27; 65–81. 113. Goldstein NS, Bhanot P, Odish E, Hunter S. Hyperplasticlike colon polyps that preceded microsatellite unstable adenocarcinomas. Am. J. Clin. Pathol. 2003; 119; 778–796. 114. O’Brien MJ, Yang S, Clebanoff JL et al. Hyperplastic (serrated) polyps of the colorectum. Relationship of CpG island methylator phenotype and K-ras mutation to location and histologic subtype. Am. J. Surg. Pathol. 2004; 28; 423–434. 115. Jass JR, Walsh MD, Barker M, Simms LA, Young J, Leggett BA. Distinction between familial and sporadic forms of colorectal cancer showing DNA microsatellite instability. Eur. J. Cancer 2002; 38; 858–866. 116. O’Brien MJ, Yang S, Mack C et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am. J. Surg. Pathol. 2006; 30; 1491–1501. 117. Jass JR. Colorectal adenomas in surgical specimens from subjects with hereditary non-polyposis colorectal cancer. Histopathology 1995; 27; 263–267. 118. Bonte H, Flejou J-F. Patterns of expression of MMR proteins in serrated adenomas and other polyps of the colorectum. Gut 2003; 52; 611 (letter). 119. Goldstein NS. Small colonic microsatellite unstable adenocarcinomas and high-grade epithelial dysplasias in sessile serrated adenoma polypectomy specimens. A study of eight cases. Am. J. Clin. Pathol. 2006; 125; 132–145. 120. Jass JR, Baker K, Zlobec I et al. Advanced colorectal polyps with the molecular and morphological features of serrated polyps and adenomas: concept of a ‘fusion’ pathway to colorectal cancer. Histopathology 2006; 49; 121–131. 121. Maltzman T, Knoll K, Martinez ME et al. Ki-ras proto-oncogene mutations in sporadic colorectal adenomas: relationship to histologic and clinical characteristics. Gastroenterology 2001; 121; 302–309. 122. Hamilton SR, Aaltonen LA. World Health Organization classification of tumours. Pathology and genetics. Lyon: IARC Press 2000. 123. Park SY, Lee HS, Choe G, Chung JH, Kim WH. Clinicopathological characteristics, microsatellite instability, and expression of mucin core proteins and p53 in colorectal mucinous adenocarcinomas in relation to location. Virchows Arch. 2006; 449; 40–47. 124. Alexander J, Watanabe T, Tsung-Teh W, Rashid A, Li S, Hamilton SR. Histopathological identification of colon cancer with microsatellite instability. Am. J. Pathol. 2001; 158; 527– 535. 125. Young J, Simms LA, Biden KG et al. Features of colorectal cancers with high-level microsatellite instability occurring in familial and sporadic settings: parallel pathways of tumorigenesis. Am. J. Pathol. 2001; 159; 2107–2116. 126. Biemer-Hüttmann A-E, Walsh MD, McGuckin MA, Young J, Leggett BA, Jass JR. Mucin core protein expression in colorectal cancers with high levels of microsatellite instability indicates a novel pathway of morphogenesis. Clin. Cancer Res. 2000; 6; 1909–1916. 127. Biemer-Hüttmann A-E, Walsh MD, McGuckin MA et al. Immunohistochemical staining patterns of MUC1, MUC2, MUC4, and MUC5AC mucins in hyperplastic polyps, serrated adenomas, and traditional adenomas of the colorectum. J. Histochem. Cytochem. 1999; 47; 1039–1047. 128. Ajioka Y, Watanabe H, Jass JR. MUC1 and MUC2 mucins in flat and polypoid colorectal adenomas. J. Clin. Pathol. 1997; 50; 417–421. 2007 The Author. Journal compilation 2007 Blackwell Publishing Ltd, Histopathology, 50, 113–130. Clasification of colorectal cancer 129. Agawa S, Jass JR. Sialic acid histochemistry and the adenoma– carcinoma sequence in the colorectum. J. Clin. Pathol. 1990; 43; 527–532. 130. Symonds DA, Vickery AL. Mucinous carcinoma of the colon and rectum. Cancer 1976; 37; 1891–1900. 131. Mecklin J-P, Sipponen P, Jarvinen HJ. Histopathology of colorectal carcinomas and adenomas in cancer family syndrome. Dis. Colon Rectum 1986; 29; 849–853. 132. Jass JR, Smyrk TC, Stewart SM, Lane MR, Lanspa SJ, Lynch HT. Pathology of hereditary non-polyposis colorectal cancer. Anticancer Res. 1994; 14; 1631–1634. 133. Kim H, Jen J, Vogelstein B, Hamilton SR. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am. J. Pathol. 1994; 145; 148–156. 134. Gafa R, Maestri I, Matteuzzi M et al. Sporadic colorectal adenocarcinomas with high-frequency microsatellite instability. Cancer 2000; 89; 2025–2037. 135. Ward R, Meagher A, Tomlinson I et al. Microsatellite instability and the clinicopathological features of sporadic colorectal cancer. Gut 2001; 48; 821–829. 136. Greenson JK, Bonner JD, Ben-Yzhak O et al. Phenotype of microsatellite unstable colorectal carcinomas. Am. J. Surg. Pathol. 2003; 27; 563–570. 137. Shashidharan M, Smyrk T, Lin KM et al. Histologic comparison of hereditary nonpolyposis colorectal cancer associated with MSH2 and MLH1 and colorectal cancer from the general population. Dis. Colon Rectum 1999; 42; 722–726. 138. Jass JR, Stewart SM, Stewart J, Lane MR. Hereditary non-polyposis colorectal cancer: morphologies, genes and mutations. Mutat. Res. 1994; 290; 125–133. 139. Sasaki P, Atkin WS, Jass JR. Mucinous carcinoma of the rectum. Histopathology 1987; 11; 259–272. 140. Ajioka Y, Xing P-X, Hinoda Y, Jass JR. Correlative histochemical study providing evidence for the dual nature of human colorectal cancer mucin. Histochem. J. 1997; 29; 143– 152. 141. Jass JR, Do K-A, Simms LA et al. Morphology of sporadic colorectal cancer with DNA replication errors. Gut 1998; 42; 673–679. 142. Gibbs NM. Undifferentiated carcinoma of the large intestine. Histopathology 1977; 1; 77–84. 143. Jessurun J, Romero-Guadarrama M, Manivel JC. Medullary adenocarcinoma of the colon. clinicopathologic study of 11 cases. Hum. Pathol. 1999; 30; 843–848. 144. Wicking C, Simms LA, Evans T et al. CDX2, a human homologue of Drosophila caudal, is mutated in both alleles in a replication error positive colorectal cancer. Oncogene 1998; 17; 657–659. 145. Yamaguchi T, Iijima T, Mori T et al. Accumulation profile of frameshift mutations during development and progression of colorectal cancer from patients with hereditary nonpolyposis colorectal cancer. Dis. Colon Rectum 2006; 49; 399– 406. 146. Hinoi T, Tani M, Lucas PC et al. Loss of CDX2 expression and microsatellite instability are prominent features of large cell minimally differentiated carcinomas of the colon. Am. J. Pathol. 2001; 159; 2239–2248. 147. Connelly JH, Robey-Cafferty SS, el-Naggar AK, Cleary KR. Exophytic signet-ring cell carcinoma of the colorectum. Arch. Pathol. Lab. Med. 1991; 115; 134–136. 148. Ogino S, Odze RD, Kawasaki T et al. Correlation of pathologic features with CpG island methylator phenotype (CIMP) by 149. 150. 151. 152. 153. 154. 155. 156. 157. 158. 159. 160. 161. 162. 163. 164. 165. 129 quantitative DNA methylation analysis in colorectal cancer. Am. J. Surg. Pathol. 2006; 30; 1175–1183. Jass JR, Atkin WS, Cuzick J et al. The grading of rectal cancer: historical perspectives and a multivariate analysis of 447 cases. Histopathology 1986; 10; 437–459. Hase K, Shatney C, Johnson D, Trollope M, Vierra M. Prognostic value of tumor ‘budding’ in patients with colorectal cancer. Dis. Colon Rectum 1993; 36; 627–635. Ueno H, Murphy J, Jass JR, Mochizuki H, Talbot IC. Tumour ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology 2002; 40; 127–132. Jass JR, Ajioka Y, Allen JP et al. Assessment of invasive growth pattern and lymphocytic infiltration in colorectal cancer. Histopathology 1996; 28; 543–548. Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat. Rev. Cancer 2005; 5; 744– 749. Brabletz T, Jung A, Hermann K, Gunther K, Hohenberger W, Kirchner T. Nuclear overexpression of the oncoprotein beta-catenin in colorectal cancer is localized predominantly at the invasion front. Pathol. Res. Pract. 1998; 194; 701– 704. Kirchner T, Brabletz T. Patterning and nuclear b-catenin expression in the colonic adenoma–carcinoma sequence. Am. J. Pathol. 2000; 157; 1113–1121. Hlubek F, Jung A, Kotzor N, Kirchner T, Brabletz T. Expression of the invasion factor laminin gamma2 in colorectal carcinomas is regulated by b-catenin. Cancer Res. 2001; 61; 8089– 8093. Leeman MF, Curran S, Murray GI. New insights into the roles of matrix metalloproteinases in colorectal cancer development and progression. J. Pathol. 2003; 201; 528–534. Wong NACS, Pignatelli M. Beta-catenin—a linchpin in colorectal carcinogenesis. Am. J. Pathol. 2002; 160; 389– 401. Beiter K, Hiendlmeyer E, Brabletz T et al. B-catenin regulates the expression of tenascin-C in human colorectal tumors. Oncogene 2005; 24; 8200–8204. El-Bahrawy MA, Poulsom R, Jeffery R, Talbot I, Alison MR. The expression of E-cadherin and catenins in sporadic colorectal carcinoma. Hum. Pathol. 2001; 32; 1216–1224. Brabletz T, Spaderna S, Kolb J et al. Down-regulation of the homeodomain factor Cdx2 in colorectal cancer by collagen type I. An active role for the tumor environment in malignant tumor progression. Cancer Res. 2004; 64; 6973– 6977. Shinto E, Mochizuki H, Ueno H, Matsubara O, Jass JR. A novel classification of tumour budding in colorectal cancer based on the presence of cytoplasmic pseudo-fragments around budding foci. Histopathology 2005; 47; 25–31. Weinstein RS, Jakate SM, Dominguez JM et al. Relationship of the expression of the multidrug resistance gene product (P-glycoprotein) in human colon carcinoma to local tumor aggressiveness and lymph node metastasis. Cancer Res. 1991; 51; 2720–2726. Wright CL, Stewart ID. Histopathology and mismatch repair status of 458 consecutive colorectal carcinomas. Am. J. Surg. Pathol. 2003; 27; 1393–1406. Shinto E, Baker K, Tsuda H et al. Tumor buds show reduced expression of laminin-5 gamma 2 chain in DNA mismatch repair deficient colorectal cancer. Dis. Colon Rectum 2006; 49; 1193–1202. 2007 The Author. Journal compilation 2007 Blackwell Publishing Ltd, Histopathology, 50, 113–130. 130 J R Jass 166. Krishna M, Burgart LJ, French AJ, Moon-Tasson L, Halling KC, Thibodeau SN. Histopathologic features associated with microsatellite instability in colorectal carcinomas. Gastroenterology 1996; 110; A546. 167. Smyrk TC, Watson P, Kaul K, Lynch HT. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal cancer. Cancer 2001; 91; 2417–2422. 168. Michael-Robinson JM, Biemer-Hüttman A-E, Purdie DM et al. Tumour infiltrating lymphocytes and apoptosis are independent features in colorectal cancer stratified according to microsatellite instability status. Gut 2001; 48; 360–366. 169. O’Connell J, O’Sullivan GC, Collins JK, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J. Exp. Med. 1996; 184; 1075– 1082. 170. Graham DM, Appelman HD. Crohn’s-like lymphoid reaction and colorectal carcinoma: a potential histologic prognosticator. Mod. Pathol. 1990; 3; 332–335. 171. Dolcetti R, Viel A, Doglioni C et al. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am. J. Pathol. 1999; 154; 1805–1813. 2007 The Author. Journal compilation 2007 Blackwell Publishing Ltd, Histopathology, 50, 113–130.







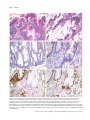

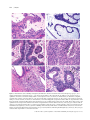














![the summary [Word]](http://s1.studyres.com/store/data/000121145_1-d789f664e59a4bf510b0d20ab68cf58c-150x150.png)











