* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chap 7 - College of Science | Oregon State University
Internal energy wikipedia , lookup
Thermal conductivity wikipedia , lookup
Heat transfer wikipedia , lookup
Thermal comfort wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Maximum entropy thermodynamics wikipedia , lookup
Thermoregulation wikipedia , lookup
R-value (insulation) wikipedia , lookup
Entropy in thermodynamics and information theory wikipedia , lookup
Adiabatic process wikipedia , lookup
Heat transfer physics wikipedia , lookup
Thermal radiation wikipedia , lookup
Temperature wikipedia , lookup
Thermodynamic system wikipedia , lookup
Thermal conduction wikipedia , lookup
Extremal principles in non-equilibrium thermodynamics wikipedia , lookup
Thermodynamic temperature wikipedia , lookup
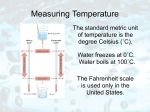
Second Law of Thermodynamics The Law of Conservation of Energy is the First Law of Thermodynamics. The Second Law of Thermodynamics has three forms. They’re different, but, really, they are all related… Form 1: Thermal energy flows spontaneously only from a region of high temperature to a region of lower temperature. It never goes the other way (spontaneously.) Remember from earlier: Temperature is a measure of the average KE of the atoms or molecules of the stuff you’re taking the temperature of. Hot and cold are relative terms! - Energy flowing out of our body makes us feel cold. - Energy flowing into our body makes us feel warm. - 55o F feels warm in winter, cold in summer! Temperature Measurement - length (of mercury column in a thermometer) due to thermal expansion - voltage (between ends of thermocouple) - electrical resistance (of a thermister) - emitted radiation (via thermograph or thermogram) - Bimetal strips are made of two metal with different thermal expansions; each side expands or contracts differently with changes in temperature. Temperature Scales Measured in oC (Celsius or centigrade), oF (Fahrenheit), or K (Kelvin). (Many others throughout history.) Celsius based on melting and boiling points of water. - Water freezes at 0oC; boils at 100oC. - There are 100 degrees between these two points. Fahrenheit based on human body temperature and freezing point of a mixture of water and salt. - Water freezes at 32oF; boils at 212oF. - There are 180 degrees between these two points. Kelvin based on absolute zero. - Also called absolute scale. - Absolute zero is the lowest possible temperature that anything can reach. - No molecular/atomic KE at absolute zero. - Absolute zero = 0 K = -273 oC - Water freezes at 273 K; boils at 373 K. - There are 100 degrees between these two points. - Kelvin is most common scientific scale used today. Form 2: It is impossible for any heat engine to be 100% efficient. There is always some thermal energy output (exhausted) from the engine that does no work. Heat Engine: Any device that uses thermal energy to do work. e.g., Gasoline, diesel, steam engines. Internal versus external combustion engines. Efficiency of a heat engine = work output / thermal energy input A Carnot Engine is a theoretical, best-possible heat engine. Its efficiency is efficiency = ( Thigh – Tlow ) / Thigh = ( Tinput – Texhaust ) / Tinput The best any heat engine (a gasoline engine for example) can do is match the Carnot engine. Gasoline engine maximum efficiency = 55% In practice, the efficiency is always less than ideal because of friction (mostly.) Gasoline engine actual efficiency = 10-15% Refrigerators, Air Conditioners, Heat Pumps are “heat engines in reverse”. Instead of moving thermal energy around to output work (motion), we put in the work (a motor drives a compressor for example) and thus move thermal energy around. Form 3: Entropy is increasing. (In any physical process within some system, the total entropy of the system either stays constant or increases. Mostly, it increases.) Entropy: Disorder; randomness. Ice melting is water going from an ordered, crystalline state, to a disordered, liquid state. We can put the water back into an ordered state by refreezing it (in ice cube trays for example!) but to do this, we must run a refrigerator that creates “waste” heat that increases the disorder of the air in the room (kitchen!) - The increase in the entropy of the room will always be greater than the decrease in the entropy of the freezing ice cubes! The fact that entropy always increases (in spontaneous reactions) gives us our perception of time moving forward. The fact that entropy always increases leads some to (incorrectly) believe that evolution must be impossible. (Evolution does not violate the second law of thermodynamics.) The fact that entropy always increases reaffirms some people’s belief in God.